Efficacy evaluation of drug-coated balloon therapy in treating patients with atherosclerotic occlusive disease of lower extremity
-
摘要:目的 探讨药物涂层球囊治疗下肢动脉粥样硬化闭塞性疾病的疗效。方法 回顾性分析接受药物涂层球囊治疗的38例下肢动脉硬化闭塞性疾病患者的术前、术中以及术后随访资料。结果 本研究38例患者患肢血管均再通成功,术后随访期间均未发生与手术相关或其他原因导致的死亡、截肢、靶病变血栓形成等并发症,无需要手术再干预患者,随访率100.00%。术后6个月、1年的Ⅰ期通畅率为84.21%、73.68%。所有患者术后不同时点的踝肱指数(ABI)、行走距离、Rutherford分级均较术前显著改善(P < 0.05)。结论 药物涂层球囊治疗下肢动脉粥样硬化闭塞性疾病的疗效显著。Abstract:Objective To explore the efficacy of drug-coated balloon therapy in treatment of patients with atherosclerotic occlusive disease of lower extremity.Methods The preoperative, intra-operative and postoperative follow-up data of 38 patients with arteriosclerosis occlusive disease of lower extremity treated by drug-coated balloon therapy were retrospectively analyzed.Results All the 38 patients had successful revascularization of affected limbs. During the follow-up period, no complications such as death, amputation, thrombosis of target lesions and other complications associated with surgery occurred. No patients with further intervention were observed, and the follow-up rate was 100.00%. The patency rate of stage Ⅰ was 84.21% and 73.68% at 6 months and 1 year after operation. Ankle Brachial Index (ABI), walking distance and Rutherford grading of all patients at different time points after operation were significantly improved when compared with those before operation (P < 0.05).Conclusion Drug-coated balloon therapy is effective in the treatment of patients with atherosclerotic occlusive diseases of lower extremity.
-
-
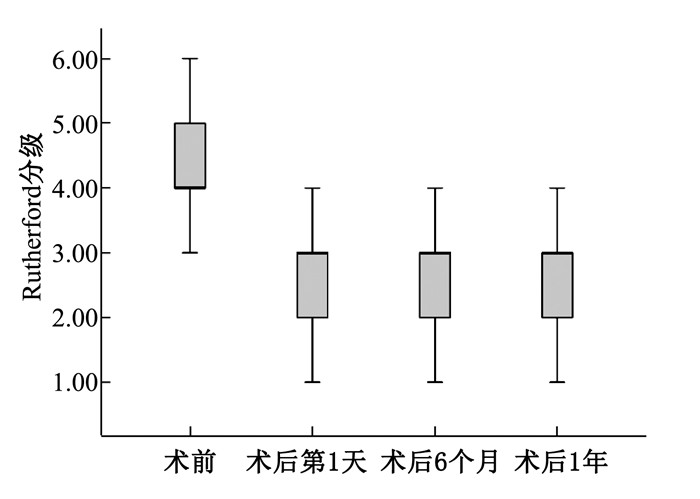
表 1 药物涂层球囊手术前后ABI、行走距离及Rutherford分级比较(x±s)
指标 时点 水平 95% CI 踝肱指数 术前 0.44±0.12 0.39~0.47 术后第1天 0.86±0.15* 0.80~0.90 术后6个月 0.78±0.16* 0.74~0.84 术后1年 0.76±0.14* 0.71~0.80 行走距离/m 术前 108.55±11.68 71.85~145.26 术后6个月 789.47±104.10* 456.15~1 122.80 术后1年 668.68±68.57* 383.19~954.18 Rutherford分级 术前 4(4,5) — 术后第1天 3(2,3)* — 术后6个月 3(2,3)* — 术后1年 3(2,3)* — 患者术后第1天未能下地行走,未统计行走距离; 患者Rutherford分级以中位数表示。与术前比较, *P < 0.05。 -
[1] Willey J, Mentias A, Vaughan-Sarrazin M, et al. Epidemiology of lower extremity peripheral artery disease in veterans[J]. J Vasc Surg, 2018, 68(2): 527-535. doi: 10.1016/j.jvs.2017.11.083
[2] Nehler M R, Duval S, Diao L H, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population[J]. J Vasc Surg, 2014, 60(3): 686-695. doi: 10.1016/j.jvs.2014.03.290
[3] Kibrik P, Victory J, Patel R, et al. A real-world experience of drug eluting and non-drug eluting stents in lower extremity peripheral arterial disease[J]. Vascular, 2019: 17085381. http://www.sciencedirect.com/science/article/pii/S0741521418311637
[4] Ramaiah V, Gammon R, Kiesz S, et al. Midterm outcomes from the TALON Registry: treating peripherals with SilverHawk: outcomes collection[J]. J Endovasc Ther, 2006, 13(5): 592-602. doi: 10.1583/05-1780MR.1
[5] Shammas N W, Dippel E J, Coiner D, et al. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry[J]. J Endovasc Ther, 2008, 15(3): 270-276. doi: 10.1583/08-2397.1
[6] Schlager O, Gschwandtner M E, Willfort-Ehringer A, et al. Drug coated balloons in the superficial femoral artery[J]. J Cardiovasc Surg (Torino), 2018, 59(1): 60-69.
[7] Schneider P A, Laird J R, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon[J]. J Am Coll Cardiol, 2019, 73(20): 2550-2563. doi: 10.1016/j.jacc.2019.01.013
[8] Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010[J]. Lancet, 2012, 380(9859): 2095-2128. doi: 10.1016/S0140-6736(12)61728-0
[9] Jaff M R, White C J, Hiatt W R, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: A supplement to the inter-society consensus for the management of peripheral arterial disease (TASC Ⅱ): The TASC steering committee[J]. Catheter Cardiovasc Interv, 2015, 86(4): 611-625. doi: 10.1002/ccd.26122
[10] Cortese B, Berti S, Biondi-Zoccai G, et al. Drug-coated balloon treatment of coronary artery disease: a position paper of the Italian Society of Interventional Cardiology[J]. Catheter Cardiovasc Interv, 2014, 83(3): 427-435. doi: 10.1002/ccd.25149
[11] Schneider P A, Laird J R, Tepe G, et al. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN. PACT SFA randomized trial[J]. Circ Cardiovasc Interv, 2018, 11(1): e005891. http://europepmc.org/articles/PMC5771683/
[12] Micari A, Nerla R, Vadalà G, et al. 2-year results of paclitaxel-coated balloons for long femoropopliteal artery disease: evidence from the SFA-long study[J]. JACC Cardiovasc Interv, 2017, 10(7): 728-734. doi: 10.1016/j.jcin.2017.01.028
[13] Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN. PACT SFA randomized trial[J]. Circulation, 2015, 131(5): 495-502. doi: 10.1161/CIRCULATIONAHA.114.011004
[14] Cortese B, Granada J F, Scheller B, et al. Drug-coated balloon treatment for lower extremity vascular disease intervention: an international positioning document[J]. Eur Heart J, 2016, 37(14): 1096-1103. doi: 10.1093/eurheartj/ehv204
[15] Dake M D, Ansel G M, Jaff M R, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results[J]. Circ Cardiovasc Interv, 2011, 4(5): 495-504. doi: 10.1161/CIRCINTERVENTIONS.111.962324
[16] Dake M D, Ansel G M, Jaff M R, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies[J]. J Am Coll Cardiol, 2013, 61(24): 2417-2427. doi: 10.1016/j.jacc.2013.03.034
[17] Scheinert D, Duda S, Zeller T, et al. The LEVANT Ⅰ (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty[J]. JACC Cardiovasc Interv, 2014, 7(1): 10-19. doi: 10.1016/j.jcin.2013.05.022
[18] Bausback Y, Willfort-Ehringer A, Sievert H, et al. Six-month results from the initial randomized study of the ranger paclitaxel-coated balloon in the femoropopliteal segment[J]. J Endovasc Ther, 2017, 24(4): 459-467. doi: 10.1177/1526602817710770
[19] Steiner S, Willfort-Ehringer A, Sievert H, et al. 12-Month Results From the First-in-Human Randomized Study of the Ranger Paclitaxel-Coated Balloon for Femoropopliteal Treatment[J]. JACC Cardiovasc Interv, 2018, 11(10): 934-941. doi: 10.1016/j.jcin.2018.01.276
[20] Albrecht T, Speck U, Baier C, et al. Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine[J]. Invest Radiol, 2007, 42(8): 579-585. doi: 10.1097/RLI.0b013e31804f5a60
-
期刊类型引用(11)
1. 杨晓杰,宋俊霞,张丽丽. 焦作地区妊娠晚期生殖道B族溶血性链球菌感染流行情况及对妊娠结局的影响. 医药论坛杂志. 2024(08): 864-868 .  百度学术
百度学术
2. 龚梅,崔莹,黄海汛. 孕妇孕晚期生殖道B群链球菌感染的现状及危险因素研究. 标记免疫分析与临床. 2024(04): 653-657 .  百度学术
百度学术
3. 吴帅,姚艺真,吴岩,泰淑红. 妊娠期孕妇感染B族溶血性链球菌的影响因素及其列线图模型构建. 华南预防医学. 2024(07): 626-630 .  百度学术
百度学术
4. 张碧慧,朱晓宁,李艳,苏芳,卫小静,曹彩萍. B族链球菌感染胎膜早破孕妇宫颈阴道分泌物IGF-1的表达及与妊娠结局的关系. 保健医学研究与实践. 2024(11): 67-72 .  百度学术
百度学术
5. 乔木,韩雁雁,姚文秀. 25-羟维生素D和乳酸脱氢酶检测B族溶血性链球菌感染新生儿的临床意义. 实用临床医药杂志. 2023(04): 44-47+65 .  本站查看
本站查看
6. 黄燕,王明英,冯军艳,张捷. 妊娠期B族溶血性链球菌感染的影响因素研究. 中国全科医学. 2022(09): 1118-1122 .  百度学术
百度学术
7. 赵建萍,林松洋,王雪侠,孙园圆,卢雪,胡明明. B族链球菌核酸检测试剂盒临床应用对比评价. 河南预防医学杂志. 2022(07): 552-555 .  百度学术
百度学术
8. 莫裕晓,李欣欣,李琰. 妊娠期B族链球菌感染的影响因素及妊娠结局分析. 保健医学研究与实践. 2022(07): 46-49 .  百度学术
百度学术
9. 郭海红,王永凯,郭丽丽. 妊娠期B族溶血性链球菌对孕妇凝血功能阴道微生态及妊娠结局的影响. 河北医学. 2022(08): 1375-1379 .  百度学术
百度学术
10. 刘园园. 阴道用药对妊娠期B族链球菌阳性并阴道微环境异常孕妇的临床研究. 临床研究. 2022(10): 58-61 .  百度学术
百度学术
11. 余蓉,葛玉东. 500例孕晚期妇女阴道或直肠拭子GBS定植率及GBS感染影响因素分析. 临床研究. 2021(12): 132-135 .  百度学术
百度学术
其他类型引用(1)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
