Expression of circRNA ANKS1B and upstream transcription factor 1 in breast cancer tissues and its clinical significance
-
摘要:目的
探讨乳腺癌组织中环状RNA ANKS1B(CircANKS1B)及上游转录因子1(USF1)的表达及其临床意义。
方法选取90例乳腺癌患者作为研究对象。采用免疫组织化学检测组织中USF1蛋白表达。采用荧光定量聚合酶链反应检测组织中CircANKS1B及USF1 mRNA的表达。分析癌组织中CircANKS1B与USF1 mRNA的相关性。分析CircANKS1B及USF1 mRNA表达与乳腺癌临床病理特征的关系。分析CircANKS1B及USF1 mRNA的表达与乳腺癌预后的关系。利用单因素及多因素COX比例风险模型分析乳腺癌预后的独立影响因素。
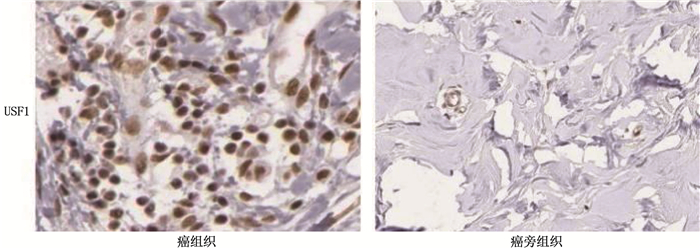
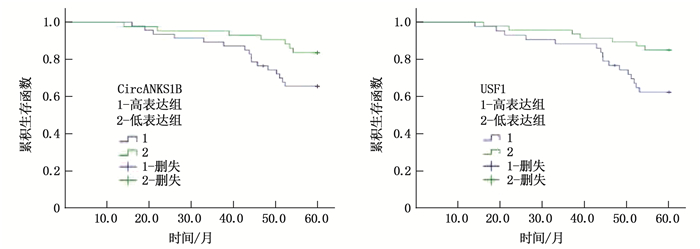
结果乳腺癌组织中USF1棕黄色阳性表达主要位于细胞核。乳腺癌组织中USF1蛋白表达阳性率为86.67%(78/90), 高于癌旁组织的15.56%(14/90), 差异有统计学意义(P<0.001)。乳腺癌组织中CircANKS1B与USF1 mRNA的相对表达量分别为(4.12±0.68)、(6.35±1.21), 高于癌旁组织的(1.23±0.46)、(1.57±0.59), 差异有统计学意义(P<0.001)。乳腺癌组织CircANKS1B与USF1 mRNA表达呈显著正相关(r=0.604, P<0.001)。CircANKS1B与USF1 mRNA表达在不同肿瘤分期、淋巴结转移及是否三阴性乳腺癌方面比较, 差异具有统计学意义(P<0.05)。CircANKS1B高表达患者中位生存时间为52.62个月(95%CI: 49.44~56.35), 短于CircANKS1B低表达患者的56.78个月(95%CI: 52.46~57.05), 差异有统计学意义(P=0.045)。USF1 mRNA低表达患者中位生存时间为52.53个月(95%CI: 48.83~56.23), 短于USF1 mRNA低表达患者的56.75个月(95%CI: 54.07~59.43), 差异有统计学意义(P=0.014)。CircANKS1B高表达、USF1 mRNA高表达、肿瘤分期Ⅲ期及合并淋巴结转移是乳腺癌预后的独立影响因素。
结论乳腺癌组织中CircANKS1B及USF1表达升高,二者与肿瘤TNM分期、淋巴结转移有关,可作为新的乳腺癌预后标志物。
-
关键词:
- 乳腺癌 /
- 环状RNA ANKS1B /
- 上游转录因子1 /
- 生物标志物
Abstract:ObjectiveTo investigate the expression and clinical significance of CircRNA ANKS1B (CircANKS1B) and upstream transcription factor 1 (USF1) in breast cancer tissues.
MethodsA total of 90 patients with breast cancer were selected as subjects. USF1 protein expression in tissues was detected by immunohistochemistry. The expression of CircANKS1B and USF1 mRNA in tissues was detected by real-time quantitative polymerase chain reaction. The correlation between CircANKS1B and USF1 mRNA in cancer tissues was analyzed. The relationships of CircANKS1B and USF1 mRNA expression with clinicopathologic features of breast cancer were analyzed. The relationships of CircANKS1B and USF1 mRNA expression with prognosis of breast cancer were analyzed. Univariate and multivariate COX proportional risk models were used to analyze the independent influencing factors of the prognosis of breast cancer.
ResultsThe brownish-yellow expression of USF1 in breast cancer tissues was mainly located in the nucleus. The positive rate of USF1 protein expression in breast cancer tissues was 86.67% (78/90), which was significantly higher than 15.56% (14/90) in adjacent tissue (P < 0.001). The relative expression levels of CircANKS1B and USF1 mRNA in the breast cancer tissues were (4.12±0.68) and (6.35±1.21), respectively, which were significantly higher than (1.23± 0.46) and (1.57±0.59) in the adjacent tissue (P < 0.001). There was a significant positive correlation between CircANKS1B and USF1 mRNA expression in breast cancer tissues (r=0.604, P < 0.001). The expression of CircANKS1B and USF1 mRNA showed statistically significant differences in different tumor stages, lymph node metastasis and triple-negative breast cancer (P < 0.05). The median survival time of patients with high CircANKS1B expression was 52.62 months (95%CI, 49.44 to 56.35), which was significantly shorter than 56.78 months (95%CI: 52.46 to 57.05) in patients with low CircANKS1B expression (P=0.045). The median survival time of patients with low expression of USF1 mRNA was 52.53 months (95%CI, 48.83 to 56.23), which was significantly shorter than 56.75 months (95%CI, 54.07 to 59.43) of patients with low USF1 mRNA expression (P=0.014). CircANKS1B high expression, USF1 mRNA high expression, tumor stage Ⅲ and lymph node metastasis are independent influencing factors of the prognosis of breast cancer.
ConclusionThe expressions of CircANKS1B and USF1 are increased in breast cancer tissues, and correlated with TNM stage, lymph node metastasis, and are new breast cancer prognostic tumor markers.
-
Keywords:
- breast cancer /
- circRNA ANKS1B /
- upstream transcription factor 1 /
- biomarker
-
-
表 1 CircANKS1B、USF1 mRNA表达与乳腺癌临床病理特征的关系(x±s)
临床参数 分类 n CircANKS1B χ2 P USF1 mRNA χ2 P 年龄 <60岁 38 3.99±0.73 1.587 0.116 6.14±1.42 1.328 0.188 ≥60岁 52 4.22±0.64 6.50±1.15 肿瘤直径 ≥2 cm 37 4.25±0.75 1.543 0.126 6.46±1.24 0.740 0.462 <2 cm 53 4.03±0.60 6.27±1.17 组织学分型 黏液腺癌 21 4.15±0.69 0.031 0.969 6.31±1.23 0.011 0.989 浸润性小叶癌 22 4.10±0.67 6.36±1.22 浸润性导管癌 47 4.12±0.64 6.35±1.18 组织学分级 Ⅰ~Ⅱ级 60 4.10±0.61 0.409 0.483 6.19±1.14 1.812 0.073 Ⅲ级 30 4.16±0.74 6.67±1.27 肿瘤分期 Ⅰ~Ⅱ期 62 3.63±0.60 10.478 <0.001 5.55±1.03 9.700 <0.001 Ⅲ期 28 5.18±0.75 8.12±1.42 淋巴结转移 有 57 3.72±0.64 7.263 <0.001 7.69±1.15 13.210 <0.001 无 33 4.81±0.76 4.04±1.44 三阴性乳腺癌 是 18 4.56±0.70 3.162 <0.001 7.67±1.26 5.201 <0.001 否 72 4.01±0.65 6.02±1.19 表 2 影响乳腺癌预后的单因素Cox回归分析
因素 赋值 β SE Wald χ2 P HR 95%CI 年龄 1=≥60岁, 0=<60岁 0.141 0.130 1.176 0.642 1.151 0.892~1.486 肿瘤大小 1=≥2 cm, 0=<2 cm 0.174 0.149 1.364 0.514 1.191 0.889~1.594 病理类型 1=浸润性小叶癌及导管癌, 0=黏液腺癌 0.259 0.190 1.858 0.319 1.296 0.892~1.880 三阴性 1=是, 0=否 0.224 0.187 1.435 0.441 1.251 0.867~1.805 组织学分级 1=Ⅲ级, 0=Ⅰ~Ⅱ级 0.249 0.212 1.283 0.309 1.380 0.847~1.944 淋巴结转移 1=有, 0=无 0.471 0.151 8.249 <0.001 1.352 0.913~1.882 肿瘤分期 1=Ⅲ期, 0=Ⅰ~Ⅱ期 0.566 0.196 9.276 <0.001 1.730 1.434~2.099 CircANKS1B 1=高表达, 0=低表达 0.641 0.181 11.667 <0.001 2.041 1.813~2.463 USF1 mRNA 1=高表达, 0=低表达 0.717 0.214 12.696 <0.001 2.060 1.921~2.279 表 3 影响乳腺癌患者预后的多因素Cox回归分析
因素 β SE Wald χ2 P HR 95%CI 淋巴结转移 0.970 0.420 5.339 0.006 2.637 1.158~6.008 肿瘤分期 0.680 0.235 8.373 <0.001 1.973 1.245~3.129 CircANKS1B 0.910 0.280 10.563 <0.001 2.484 1.435~4.301 USF1 mRNA 0.565 0.231 8.287 <0.001 1.660 1.236~3.058 -
[1] 张雪, 董晓平, 管雅喆, 等. 女性乳腺癌流行病学趋势及危险因素研究进展[J]. 肿瘤防治研究, 2021, 48(1): 87-92. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLFY202101017.htm [2] 李颖, 姜达, 刘晓丽, 等. 晚期乳腺癌原发病灶切除对患者预后的影响[J]. 中华肿瘤杂志, 2021, 43(8): 878-882. https://www.cnki.com.cn/Article/CJFDTOTAL-XNGF202004003.htm [3] 刘德慧, 严玉兰. 环状RNA在肺癌诊断及预后中的研究进展[J]. 实用临床医药杂志, 2021, 25(13): 124-128. doi: 10.7619/jcmp.20211851 [4] ZENG K X, HE B S, YANG B B, et al. The pro-metastasis effect of circANKS1B in breast cancer[J]. Mol Cancer, 2018, 17(1): 160. doi: 10.1186/s12943-018-0914-x
[5] BERNARDINI A, LORENZO M, CHAVES-SANJUAN A, et al. The USR domain of USF1 mediates NF-Y interactions and cooperative DNA binding[J]. Int J Biol Macromol, 2021, 193(Pt A): 401-413.
[6] COSTA L, CORRE S, MICHEL V, et al. USF1 defect drives p53 degradation during Helicobacter pylori infection and accelerates gastric carcinogenesis[J]. Gut, 2020, 69(9): 1582-1591. doi: 10.1136/gutjnl-2019-318640
[7] COUGHLIN S S. Epidemiology of breast cancer in women[J]. Adv Exp Med Biol, 2019, 1152: 9-29.
[8] 邢德纯, 王霞, 孙锁柱, 等. 分子诊断技术在乳腺癌检测中的最新进展[J]. 生物化学与生物物理进展, 2020, 47(3): 224-232. doi: 10.16476/j.pibb.2019.0253 [9] CAO M J, ZHANG L S, WANG J H, et al. Identifying circRNA-associated-ceRNA networks in retinal neovascularization in mice[J]. Int J Med Sci, 2019, 16(10): 1356-1365. doi: 10.7150/ijms.35149
[10] LI D Z, YANG R X, YANG L, et al. circANKS1B regulates FOXM1 expression and promotes cell migration and invasion by functioning as a sponge of the miR-149 in colorectal cancer[J]. Onco Targets Ther, 2019, 12: 4065-4073. doi: 10.2147/OTT.S201310
[11] TAO L J, PAN X Y, WANG J W, et al. Circular RNA circANKS1B acts as a sponge for miR-152-3p and promotes prostate cancer progression by upregulating TGF-α expression[J]. Prostate, 2021, 81(5): 271-278. doi: 10.1002/pros.24102
[12] YAN J W, XU H Y. Regulation of transforming growth factor-beta1 by circANKS1B/miR-515-5p affects the metastatic potential and cisplatin resistance in oral squamous cell carcinoma[J]. Bioengineered, 2021, 12(2): 12420-12430. doi: 10.1080/21655979.2021.2005221
[13] ONADEKO O T, OKUNOWO W O, IMAGA N O A, et al. Polymorphism rs3737787 of Upstream Stimulatory Factor 1 gene is associated with serum lipid phenotype in Nigerian population[J]. Mol Cell Probes, 2021, 55: 101687. doi: 10.1016/j.mcp.2020.101687
[14] TAGHIZADEH E, MIRZAEI F, JALILIAN N, et al. A novel mutation in USF1 gene is associated with familial combined hyperlipidemia[J]. IUBMB Life, 2020, 72(4): 616-623. doi: 10.1002/iub.2186
[15] WANG J T, GU J S, YOU A W, et al. The transcription factor USF1 promotes glioma cell invasion and migration by activating lncRNA HAS2-AS1[J]. Biosci Rep, 2020, 40(8): BSR20200487. doi: 10.1042/BSR20200487
[16] ALPER M, AYDEMIR T, KÖÇKAR F. USF1 suppresses expression of fibrillar type Ⅰ, Ⅱ, and Ⅲ collagen and pNP adamts-3 in osteosarcoma cells[J]. Mol Biol, 2021, 55(4): 580-588. doi: 10.1134/S0026893321030031
[17] LI Z L, JIANG X M, HUANG L N, et al. Up-regulation of ZFAS1 indicates dismal prognosis for cholangiocarcinoma and promotes proliferation and metastasis by modulating USF1 via miR-296-5p[J]. J Cell Mol Med, 2019, 23(12): 8258-8268. doi: 10.1111/jcmm.14698
[18] WANG D D, JIN X Y, LEI M X, et al. USF1-ATRAP-PBX3 axis promote breast cancer glycolysis and malignant phenotype by activating AKT/mTOR signaling[J]. Int J Biol Sci, 2022, 18(6): 2452-2471. doi: 10.7150/ijbs.69134
[19] CHEN L, HAN S J, LI Y, et al. SEZ6L2, regulated by USF1, accelerates the growth and metastasis of breast cancer[J]. Exp Cell Res, 2022, 417(1): 113194. doi: 10.1016/j.yexcr.2022.113194
[20] YE H, LIU X J, HUI Y, et al. USF1 gene polymorphisms may associate with the efficacy and safety of chemotherapy based on paclitaxel and prognosis in the treatment of ovarian cancer[J]. Neoplasma, 2018, 65(1): 153-160. doi: 10.4149/neo_2018_170322N205
[21] MOONEY S M, TALEBIAN V, JOLLY M K, et al. The GRHL2/ZEB feedback loop-a key axis in the regulation of EMT in breast cancer[J]. J Cell Biochem, 2017, 118(9): 2559-2570. doi: 10.1002/jcb.25974
-
期刊类型引用(6)
1. 王腊梅,白斌芳. 冠心病合并高脂血症患者应用荷丹胶囊联合瑞舒伐他汀钙片治疗的临床效果观察. 心血管病防治知识. 2024(19): 38-41 .  百度学术
百度学术
2. 张凯. 依折麦布结合瑞舒伐他汀钙对冠心病患者血清hs-CRP水平及颈动脉粥样硬化斑块的影响分析. 贵州医药. 2023(04): 557-558 .  百度学术
百度学术
3. 杨晓凯. 不同剂量瑞舒伐他汀钙辅助治疗老年慢性心衰患者的疗效比较. 辽宁医学杂志. 2023(04): 23-26 .  百度学术
百度学术
4. 王宝典,张慧. 瑞舒伐他汀钙联合美托洛尔治疗冠心病合并心力衰竭患者的效果. 深圳中西医结合杂志. 2022(10): 92-95 .  百度学术
百度学术
5. 许西骞. 高剂量瑞舒伐他汀辅助治疗对冠心病合并心衰患者心功能及血清BNP水平的影响. 中国现代药物应用. 2022(20): 121-125 .  百度学术
百度学术
6. 单星星. 瑞舒伐他汀钙片联合琥珀酸美托洛尔缓释片治疗老年冠心病合并心力衰竭的疗效观察. 中西医结合心血管病电子杂志. 2022(03): 11-13 .  百度学术
百度学术
其他类型引用(1)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号