Correlation of prognostic nutritional index and related indicators with prognosis of postoperative patients with glioma
-
摘要:目的
探讨预后营养指数(PNI)及其他临床指标与脑胶质瘤术后患者预后的相关性。
方法回顾性选取行手术治疗的151例脑胶质瘤患者作为研究对象, 收集患者临床资料并进行随访。绘制受试者工作特征(ROC)曲线分析不同指标对患者预后的预测效能,采用Kaplan-Meier法绘制生存曲线,通过Log-rank检验和Cox回归分析探讨患者预后的影响因素。
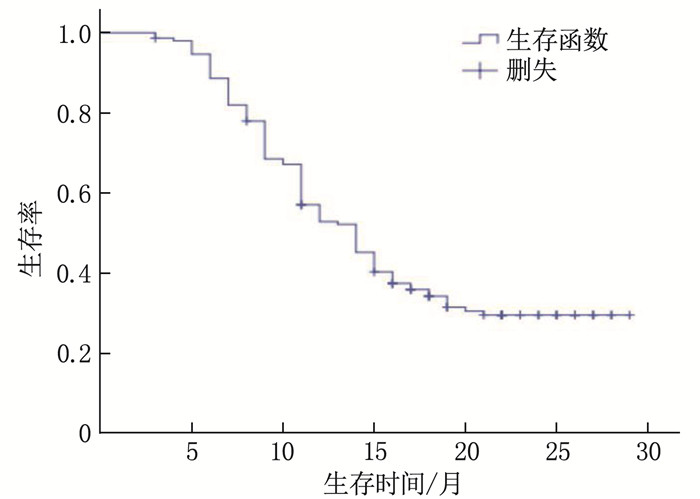
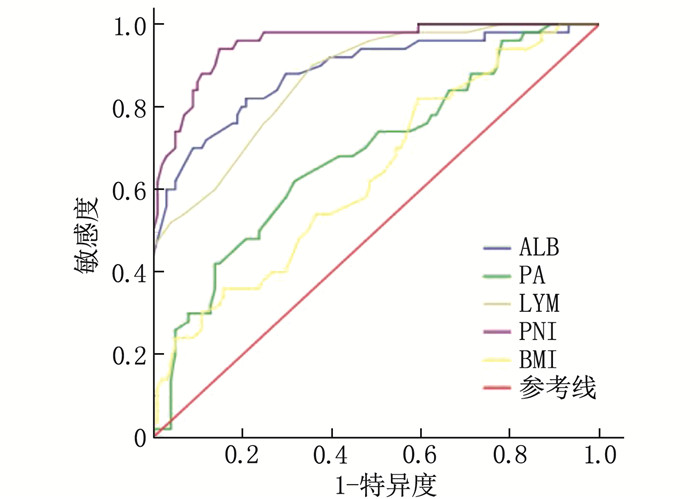
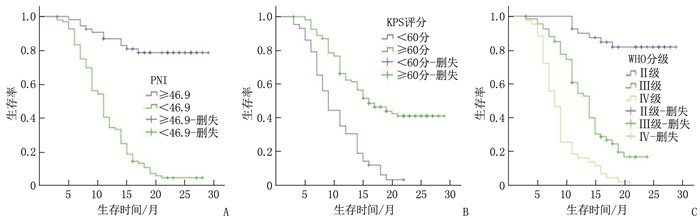
结果151例患者中位随访时间为13个月,平均生存周期21.89个月,随访期内死亡101例(占66.89%)。白蛋白(ALB)、前白蛋白(PA)、淋巴细胞计数(LYM)、PNI、体质量指数(BMI)和C反应蛋白(CRP)预测患者预后的最佳截断值分别为36.45 g/L、206.0 mg/L、1.85×109/L、46.9、21.45 kg/m2和8.55 mg/L, 曲线下面积分别为0.885、0.683、0.867、0.955、0.635和0.646。单因素分析显示, 世界卫生组织(WHO)中枢神经系统肿瘤分级(简称WHO分级)、术式、术前卡氏功能状态(KPS)评分、ALB、PA、LYM、BMI、CRP和PNI均为脑胶质瘤患者预后的影响因素(P < 0.05)。多因素Cox回归分析显示, PNI≥46.9、术前KPS评分≥60分和WHO分级Ⅱ级、Ⅲ级(相对于Ⅳ级)均为脑胶质瘤患者预后的保护因素(HR=0.925、0.964、0.122、0.577, P < 0.05)。WHO分级Ⅱ级、Ⅲ级、Ⅳ级患者的1年生存率分别为85.37%、50.75%、16.28%, PNI < 46.9和PNI≥46.9患者的1年生存率分别为34.38%和87.27%, 术前KPS评分 < 60分和KPS评分≥60分患者的1年生存率分别为30.23%和62.96%。
结论脑胶质瘤患者的临床预后较差, PNI、术前KPS评分和WHO分级均与患者预后相关。
-
关键词:
- 脑胶质瘤 /
- 营养指数 /
- 预后 /
- 中枢神经系统肿瘤分级 /
- 卡氏功能状态评分
Abstract:ObjectiveTo discuss the correlation of prognostic nutritional index (PNI) and related indicators with prognosis of postoperative patients with glioma.
MethodsA total of 151 patients with glioma were retrospectively selected as study objects, and relevant clinical data were collected and the follow-up was conducted. Receiver operating characteristic (ROC) curve was drawn to analyze the efficacy of different indicators in predicting prognosis. Kaplan-Meier method was used to draw the survival curve, and Log-rank test and Cox regression were used to analyze the influencing factors of prognosis.
ResultsOf the 151 patients, the median follow-up duration was 13 months, and the average survival period was 21.9 months. The death rate during the follow-up period was 66.89%(101 cases). The best cutoff values of albumin (ALB), prealbumin (PA), lymphocyte (LYM), PNI, body mass index (BMI) and C-reactive protein (CRP) in predicting the prognosis of patients were 36.45 g/L, 206.0 mg/L and 1.85× 109/L, 46.9, 21.45 kg/m2 and 8.55 mg/L, respectively, with areas under the curve of 0.885, 0.683, 0.867, 0.955, 0.635 and 0.646, respectively. Univariate analysis showed that World Health Organization (WHO) tumor grading for central nervous system (short for WHO grading), operation mode, preoperative Karnofsky Performance Status (KPS) score, ALB, PA, LYM, BMI, CRP and PNI were the influencing factors of prognosis in glioma patients (P < 0.05). COX multivariate analysis showed that PNI ≥ 46.9, preoperative KPS score ≥60 and WHO grade Ⅱ and Ⅲ (compared with grade Ⅳ) are the protective factors for the prognosis of patients with glioma(HR=0.925, 0.964, 0.122 and 0.577 respectively, P < 0.05). The one-year survival rates of patients with grade Ⅱ, Ⅲ and Ⅳ of WHO were 85.37%, 50.75 and 16.28% respectively, and the one-year survival rates of patients with PNI < 46.9 and ≥46.9 were 34.38% and 87.27% respectively. The one-year survival rates of patients with preoperative KPS score < 60 and ≥60 were 30.23% and 62.96%, respectively.
ConclusionThe clinical prognosis of glioma is poor. PNI, preoperative KPS score and WHO grade are related to the clinical outcome of patients with glioma.
-
-
表 1 不同指标对患者预后的预测效能
指标 AUC 95%CI 最佳截断值 约登指数 P ALB/(g/L) 0.885 0.824~0.946 36.45 0.612 <0.001 PA/(mg/L) 0.683 0.592~0.774 206.00 0.303 <0.001 LYM/(×109/L) 0.867 0.809~0.926 1.85 0.544 <0.001 PNI 0.955 0.923~0.987 46.90 0.771 <0.001 BMI/(kg/m2) 0.635 0.542~0.729 21.45 0.226 0.007 CRP/(mg/L) 0.646 0.554~0.738 8.55 0.246 0.004 ALB: 白蛋白; PA: 前白蛋白; LYM: 淋巴细胞计数; PNI: 预后营养指数; BMI: 体质量指数; CRP: C反应蛋白。 表 2 患者临床预后影响因素的单因素分析
指标 分类 n 死亡/例 生存时间(95%CI)/月 Log-rank χ2 P 性别 男 81 49 17.35(15.27~19.44) 2.282 0.131 女 70 52 14.21(12.56~15.86) WHO分级 Ⅱ级 41 7 26.16(24.24~28.09) 91.277 <0.001 Ⅲ级 67 51 14.16(12.74~15.58) Ⅳ级 43 43 9.05(7.83~10.26) 术式 全切除术 58 19 21.89(19.61~24.17) 38.730 <0.001 部分切除术 93 82 12.69(11.32~14.07) 术前KPS评分 <60分 43 41 10.29(8.88~11.69) 32.926 <0.001 ≥60分 108 60 18.71(16.95~20.48) ALB <36.45 g/L 89 80 12.28(10.94~13.62) 43.469 <0.001 ≥36.45 g/L 62 21 22.53(20.25~24.80) PA <206.0 mg/L 88 69 13.83(12.16~15.50) 12.808 <0.001 ≥206.0 mg/L 63 32 19.60(17.30~21.91) LYM <1.85×109/L 70 65 12.08(10.80~13.36) 30.812 <0.001 ≥1.85×109/L 81 36 20.20(18.03~22.38) BMI <21.45 kg/m2 50 41 13.47(11.93~15.00) 4.554 0.033 ≥21.45 kg/m2 101 60 17.39(15.47~19.32) CRP <8.55 mg/L 99 58 17.85(15.94~19.76) 8.956 0.003 ≥8.55 mg/L 52 43 13.11(11.27~14.95) PNI <46.9 96 90 11.64(10.53~12.76) 71.030 <0.001 ≥46.9 55 11 25.14(23.08~27.21) WHO: 世界卫生组织; KPS: 卡氏功能状态; ALB: 白蛋白; PA: 前白蛋白; LYM: 淋巴细胞计数; PNI: 预后营养指数; BMI: 体质量指数; CRP: C反应蛋白。 表 3 患者预后影响因素的多因素Cox回归分析
因素 β S. E. HR(95%CI) Wald χ2 P PNI -0.078 0.024 0.925(0.882~0.970) 10.464 0.001 术前KPS评分 -0.037 0.010 0.964(0.945~0.983) 13.323 <0.001 WHO分级 Ⅱ级 -2.104 0.455 0.122(0.050~0.297) 21.402 <0.001 Ⅲ级 -0.550 0.228 0.577(0.369~0.901) 5.840 0.016 -
[1] FANGUSARO J, ONAR-THOMAS A, YOUNG POUSSAINT T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial[J]. Lancet Oncol, 2019, 20(7): 1011-1022. doi: 10.1016/S1470-2045(19)30277-3
[2] WANG Z H, GAO L, GUO X P, et al. Development and validation of a nomogram with an autophagy-related gene signature for predicting survival in patients with glioblastoma[J]. Aging, 2019, 11(24): 12246-12269. doi: 10.18632/aging.102566
[3] HU C, CHEN K N, TANG X P. Prognostic value of preoperative controlling nutritional status in patients with glioblastoma[J]. Clin Neurol Neurosurg, 2020, 198: 106129. doi: 10.1016/j.clineuro.2020.106129
[4] XIAO Y P, WEI G, MA M, et al. Association among prognostic nutritional index, post-operative infection and prognosis of stage Ⅱ/Ⅲ gastric cancer patients following radical gastrectomy[J]. Eur J Clin Nutr, 2022, 76(10): 1449-1456. doi: 10.1038/s41430-022-01120-7
[5] 《中国中枢神经系统胶质瘤诊断和治疗指南》编写组. 中国中枢神经系统胶质瘤诊断和治疗指南(2015)[J]. 中华医学杂志, 2016, 96(7): 485-509. doi: 10.3760/cma.j.issn.0376-2491.2016.07.003 [6] XU S C, TANG L, LI X Z, et al. Immunotherapy for glioma: current management and future application[J]. Cancer Lett, 2020, 476: 1-12. doi: 10.1016/j.canlet.2020.02.002
[7] MLADENOVSK M, VALKOV I, OVCHAROV M, et al. High grade glioma surgery-clinical aspects and prognosis[J]. Folia Med (Plovdiv), 2021, 63(1): 35-41. doi: 10.3897/folmed.63.e55255
[8] ALAN O, TELLI T A, BASOGLU T, et al. Impact of prognostic nutritional index on survival in recurrent glioblastoma[J]. Neurocirugia, 2022, 33(1): 15-21. doi: 10.1016/j.neucir.2020.11.005
[9] 李涛, 翟景光, 刘晓. 脑胶质瘤患者手术治疗预后不良的相关因素分析[J]. 齐齐哈尔医学院学报, 2022, 43(11): 1047-1051. doi: 10.3969/j.issn.1002-1256.2022.11.011 [10] RATHORE S, AKBARI H, DOSHI J, et al. Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma: implications for personalized radiotherapy planning[J]. J Med Imaging, 2018, 5(2): 021219.
[11] LIANG R, ZHI Y, ZHENG G, et al. Analysis of long non-coding RNAs in glioblastoma for prognosis prediction using weighted gene co-expression network analysis, Cox regression, and L1-LASSO penalization[J]. Onco Targets Ther, 2019, 12: 157-168.
[12] DEMIRELLI B, BABACAN N A, ERCELEP Ö, et al. Modified Glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, Cachexia and some inflammatory indices in metastatic gastric cancer[J]. Nutr Cancer, 2021, 73(2): 230-238. doi: 10.1080/01635581.2020.1749290
[13] WANG D, HU X, XIAO L, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC[J]. J Gastrointest Surg, 2021, 25(2): 421-427. doi: 10.1007/s11605-019-04492-7
[14] 徐日. 预后营养指数对胶质瘤患者预后的影响[J]. 分子诊断与治疗杂志, 2020, 12(2): 203-206. [15] 晁储瑞, 赵伟峰, 刘慧敏, 等. 脑胶质瘤患者术前血液学指标与预后的关系[J]. 中华实用诊断与治疗杂志, 2019, 33(11): 1105-1109. [16] SENGER A S, DINCER M, UZUN O, et al. Impact of preoperative prognostic nutritional index levels on morbidity in colorectal cancer surgery[J]. Ann Ital Chir, 2022, 92: 422-426.
[17] DING P A, GUO H H, SUN C Y, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study[J]. BMC Gastroenterol, 2022, 22(1): 121. doi: 10.1186/s12876-022-02199-9
[18] HE J, YIN H, XIA Y, et al. Prognostic nutritional index, a novel biomarker which predicts worse prognosis in diffuse large B cell lymphoma[J]. Leuk Res, 2021, 110: 106664. doi: 10.1016/j.leukres.2021.106664
[19] DEME D, TELEKES A. Prognostic importance of albumin in oncology[J]. Orv Hetil, 2018, 159(3): 96-106. doi: 10.1556/650.2018.30885
[20] KIM Y J, OH H, LEE S J, et al. Prognostic significance of the postoperative prognostic nutritional index in patients with glioblastoma: a retrospective study[J]. BMC Cancer, 2021, 21(1): 942. doi: 10.1186/s12885-021-08686-8
[21] 刘竞辉, 娄淼, 冀培刚, 等. 老年胶质瘤预后相关因素分析[J]. 中南大学学报: 医学版, 2018, 43(4): 403-409. https://www.cnki.com.cn/Article/CJFDTOTAL-HNYD201804013.htm [22] BARBAGALLO G M V, CERTO F, DI GREGORIO S, et al. Recurrent high-grade glioma surgery: a multimodal intraoperative protocol to safely increase extent of tumor resection and analysis of its impact on patient outcome[J]. Neurosurg Focus, 2021, 50(1): E20. doi: 10.3171/2020.10.FOCUS20744
-
期刊类型引用(9)
1. 冯嘉伟. 宫颈液基细胞学检查与宫颈病变临床病理分析. 实用妇科内分泌电子杂志. 2024(06): 79-81 .  百度学术
百度学术
2. 况漫,马亚琳,朱贵娟. 湖北十堰地区女性HPV感染分布及类型特征分析. 公共卫生与预防医学. 2023(01): 127-130 .  百度学术
百度学术
3. 唐绥清. 液基细胞学检查及高危HPV检测联合阴道镜检查对宫颈癌筛查的临床意义. 中国社区医师. 2021(03): 141-142 .  百度学术
百度学术
4. 付敏,陈路燕. HPV及TCT联合阴道镜活检在宫颈癌分层筛查中的临床应用价值. 当代医学. 2021(12): 74-76 .  百度学术
百度学术
5. 王熙,王丽莎,杨翠,贾立云,潘雪娇,杨会欣,马翠霞,祁麟. 石家庄市育龄女性人乳头瘤病毒感染及病毒分型情况分析. 中国性科学. 2021(05): 84-87 .  百度学术
百度学术
6. 田秋艳. 宫颈液基细胞学与人乳头瘤病毒检测在宫颈低级别上皮内病变中的相关性研究. 中国社区医师. 2021(29): 111-112 .  百度学术
百度学术
7. 柏永华,曾丽莉,纪青. 宫颈液基细胞学检查对宫颈病变的筛查价值. 中国卫生标准管理. 2020(04): 122-124 .  百度学术
百度学术
8. 刘群香,王莉,李江丽. 持续感染状态的HPV-16及HPV-58亚型在宫颈病变中的分布及危险因素. 河北医学. 2020(04): 541-545 .  百度学术
百度学术
9. 唐永红,刘涛,彭健. 人乳头瘤病毒在宫颈病变中的感染分型及其特点. 系统医学. 2020(17): 134-136 .  百度学术
百度学术
其他类型引用(6)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
