Histone demethylase KDM5A regulates the occurrence and development of acute myeloid leukemia through LncRNA TRIM52-AS1
-
摘要:目的
初步探索组蛋白去甲基化酶赖氨酸特异性去甲基化酶5(KDM5A)通过长链非编码RNA(LncRNA) TRIM52-AS1调控急性髓系白血病(AML)发生、发展的机制。
方法通过逆转录定量聚合酶链式反应(qRT-PCR)和蛋白质印迹(Western blot)检测白血病患者血清和各种白血病细胞中的KDM5A、TRIM52-AS1的表达。采用双荧光素酶报告实验检测KDM5A和TRIM52-AS1的靶向关系。通过CCK8实验检测KDM5A和TRIM52-AS1对HL-60细胞增殖的影响。通过Transwell实验检测KDM5A和TRIM52-AS1对HL-60细胞迁移的影响。
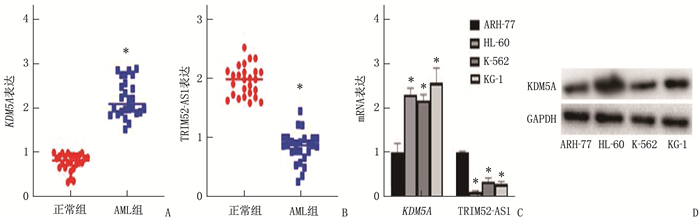
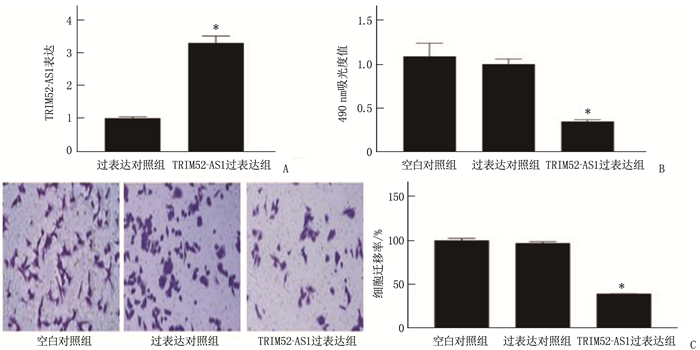
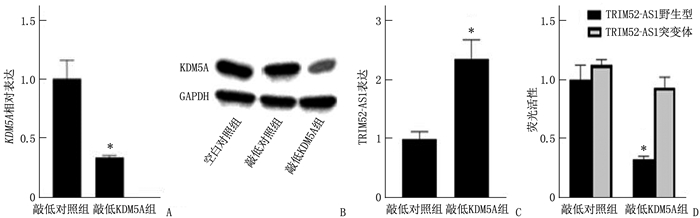
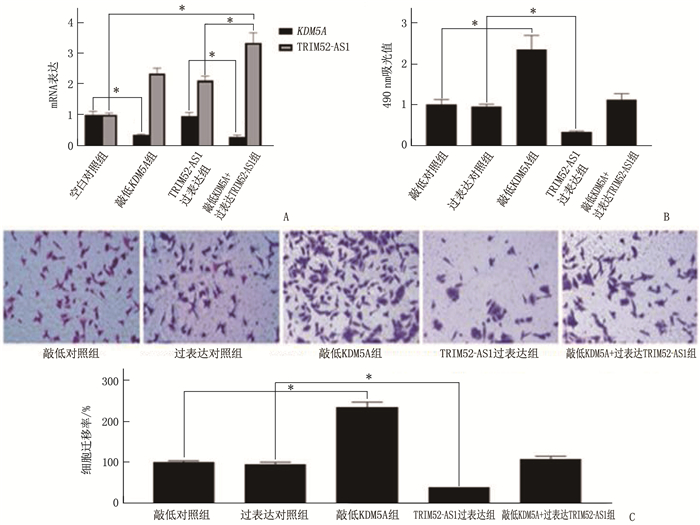
结果KDM5A在白血病患者血清和各种白血病细胞中高表达, TRIM52-AS1在白血病患者血清和各种白血病细胞中低表达(P < 0.05)。KDM5A可以靶向抑制TRIM52-AS1。过表达TRIM52-AS1可以抑制白血病细胞的增殖和迁移, KDM5A可以通过抑制TRIM52-AS1进而抑制白血病细胞的增殖和迁移(P < 0.05)。
结论组蛋白去甲基化酶KDM5A可通过抑制LncRNA TRIM52-AS1进而抑制AML的发生、发展。
-
关键词:
- 赖氨酸特异性去甲基化酶5 /
- 长链非编码RNA TRIM52-AS1 /
- 急性髓系白血病 /
- 增殖 /
- 迁移
Abstract:ObjectiveTo preliminarily explore the mechanism histone demethylase KDM5A in regulating the occurrence and development of acute myeloid leukemia (AML) through long non-coding RNA (LncRNA) TRIM52-AS1.
MethodsThe levels of KDM5A and TRIM52-AS1 were detected by real-time quantitative polymerase chain reaction (qRT-PCR) and Western blot in the serum and various leukemia cells of AML patients. The targeting relationship between KDM5A and TRIM52-AS1 was detected by dual luciferase reporter assay. The effects of KDM5A and TRIM52-AS1 on HL-60 cell proliferation were detected by CCK8 assay. The effects ofKDM5A and TRIM52-AS1 on HL-60 cell migration were detected by Transwell assay.
ResultsKDM5A was highly expressed in the serum and various leukemia cells of AML patients, while TRIM52-AS1 was lowly expressed (P < 0.05). KDM5A could targetedly inhibit TRIM52-AS1. Overexpression of TRIM52-AS1 could targetedly inhibit leukemia cell proliferation and migration, and KDM5A could inhibit leukemia cell proliferation and migration by inhibiting TRIM52-AS1 (P < 0.05).
ConclusionHistone demethylase KDM5A can inhibit the occurrence and developmen of AML by inhibiting LncRNA TRIM52-AS1.
-
-
-
[1] WEINBERG O K, HASSERJIAN R P, BARABAN E, et al. Clinical, immunophenotypic, and genomic findings of acute undifferentiated leukemia and comparison to acute myeloid leukemia with minimal differentiation: a study from the bone marrow pathology group[J]. Mod Pathol, 2019, 32(9): 1373-1385. doi: 10.1038/s41379-019-0263-3
[2] ZHOU J B, YIYING QUAH J, NG Y, et al. ASLAN003, a potent dihydroorotate dehydrogenase inhibitor for differentiation of acute myeloid leukemia[J]. Haematologica, 2020, 105(9): 2286-2297.
[3] CHEN H, HUANG Y X, ZHU X Q, et al. Histone demethylase UTX is a therapeutic target for diabetic kidney disease[J]. J Physiol, 2019, 597(6): 1643-1660. doi: 10.1113/JP277367
[4] LIU Z F, ZHANG G M, DENG M T, et al. Inhibition of lysine-specific histone demethylase 1A results in meiotic aberration during oocyte maturation in vitro in goats[J]. Theriogenology, 2020, 143: 168-178. doi: 10.1016/j.theriogenology.2019.12.011
[5] LIU Y J, WU Y K, LIU S, et al. Long non-coding RNA TRIM52-AS1 promotes growth and metastasis via miR-218-5p/ROBO1 in hepatocellular carcinoma[J]. Cancer Manag Res, 2021, 13: 547-558. doi: 10.2147/CMAR.S286205
[6] LIU Z, YAN H Y, XIA S Y, et al. Downregulation of long non-coding RNA TRIM52-AS1 functions as a tumor suppressor in renal cell carcinoma[J]. Mol Med Rep, 2016, 13(4): 3206-3212. doi: 10.3892/mmr.2016.4908
[7] 陈莉, 李青山, 张学强, 等. 髓系白血病细胞中细胞因子信号转导抑制因子1基因启动子去甲基化对细胞增殖、凋亡的影响[J]. 实用临床医药杂志, 2022, 26(8): 60-65. doi: 10.7619/jcmp.20212911 [8] 任羽, 贺爱军, 葛繁梅. nm23-H1蛋白在急性髓细胞白血病患者中的表达及其疗效[J]. 实用临床医药杂志, 2016, 20(5): 43-45, 49. doi: 10.7619/jcmp.201605013 [9] PAPAIOANNOU D, NICOLET D, OZER H G, et al. Prognostic and biologic relevance of clinically applicable long noncoding RNA profiling in older patients with cytogenetically normal acute myeloid leukemia[J]. Mol Cancer Ther, 2019, 18(8): 1451-1459. doi: 10.1158/1535-7163.MCT-18-1125
[10] ZHANG J H, ZHANG H N, WANG X H, et al. PCAT18, as a novel differentially regulated long noncoding RNA in adult acute myeloid leukemia patients revealed by next-generation sequencing[J]. Int J Lab Hematol, 2020, 42(6): 858-865. doi: 10.1111/ijlh.13305
[11] WANG Y H, LIN C C, HSU C L, et al. Distinct clinical and biological characteristics of acute myeloid leukemia with higher expression of long noncoding RNA KIAA0125[J]. Ann Hematol, 2021, 100(2): 487-498. doi: 10.1007/s00277-020-04358-y
[12] MA L, KUAI W X, SUN X Z, et al. Long noncoding RNA LINC00265 predicts the prognosis of acute myeloid leukemia patients and functions as a promoter by activating PI3K-AKT pathway[J]. Eur Rev Med Pharmacol Sci, 2018, 22(22): 7867-7876.
[13] YAN X J, QU Y. Long noncoding RNA HOXA-AS2 As a predictor of acute myeloid leukemia: clinical association between HOXA-AS2 expression and its role in leukemic cell growth[J]. Blood, 2018, 132(Suppl 1): 5131-5131.
[14] TIAN Y J, WANG Y H, XIAO A J, et al. Long noncoding RNA SBF2-AS1 act as a ceRNA to modulate cell proliferation via binding with miR-188-5p in acute myeloid leukemia[J]. Artif Cells Nanomed Biotechnol, 2019, 47(1): 1730-1737. doi: 10.1080/21691401.2019.1608221
[15] ZHAO D Z, ZHANG Q, LIU Y Q, et al. H3K4me3 demethylase Kdm5a is required for NK cell activation by associating with p50 to suppress SOCS1[J]. Cell Rep, 2020, 30(7): 2460. doi: 10.1016/j.celrep.2020.01.104
[16] WANG C D, WANG J, LI J, et al. KDM5A controls bone morphogenic protein 2-induced osteogenic differentiation of bone mesenchymal stem cells during osteoporosis[J]. Cell Death Dis, 2016, 7(8): e2335. doi: 10.1038/cddis.2016.238
[17] GE W S, SHI L, ZHOU Y S, et al. Inhibition of osteogenic differentiation of human adipose-derived stromal cells by retinoblastoma binding protein 2 repression of RUNX2-activated transcription[J]. Stem Cells, 2011, 29(7): 1112-1125. doi: 10.1002/stem.663
[18] QI X L, ZHANG D H, WU N, et al. ceRNA in cancer: possible functions and clinical implications[J]. J Med Genet, 2015, 52(10): 710-718. doi: 10.1136/jmedgenet-2015-103334
-
期刊类型引用(1)
1. 郝玉青,孙宇,王巧改. 血清同型半胱氨酸、乳酸脱氢酶、白细胞介素-17水平在急性白血病中的变化及意义. 实用临床医药杂志. 2024(15): 115-119 .  本站查看
本站查看
其他类型引用(0)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
