Effectiveness and external validation of a prognostic prediction model for diffuse large B-cell lymphoma
-
摘要:目的
分析影响弥漫性大B细胞淋巴瘤(DLBCL)患者预后的相关因素, 并建立列线图预测DLBCL患者的预后。
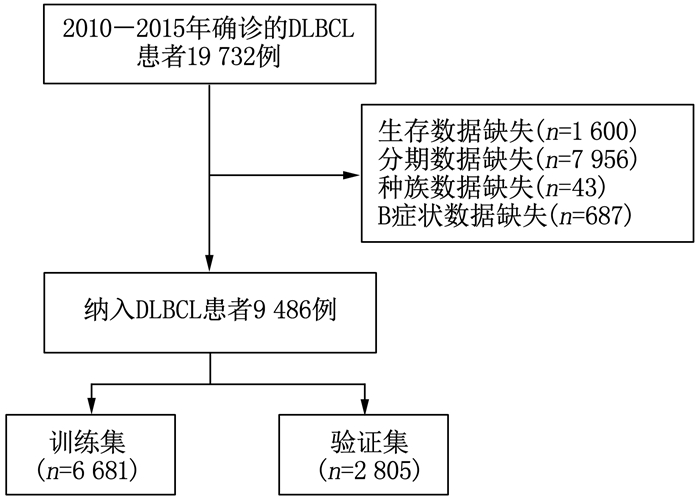
方法利用美国国家癌症研究所监测、流行病学和最终结果数据库(SEER数据库)提取9 486例DLBCL患者的基线资料, 将SEER数据按照7:3比例随机分为SEER训练集6 681例和SEER验证集2 805例。采用单因素、多因素Cox回归分析确定影响DLBCL患者预后的危险因素, 并在此基础上建立列线图; 采用一致性指数(C-index)、受试者工作特征(ROC)曲线的曲线下面积(AUC)以及校准曲线评价模型的区分度及其预测效能。选取2013年1月-2017年12月本院血液内科及肿瘤科的120例DLBCL患者作为外部验证集, 对列线图进行外部验证并分析其影响因素。
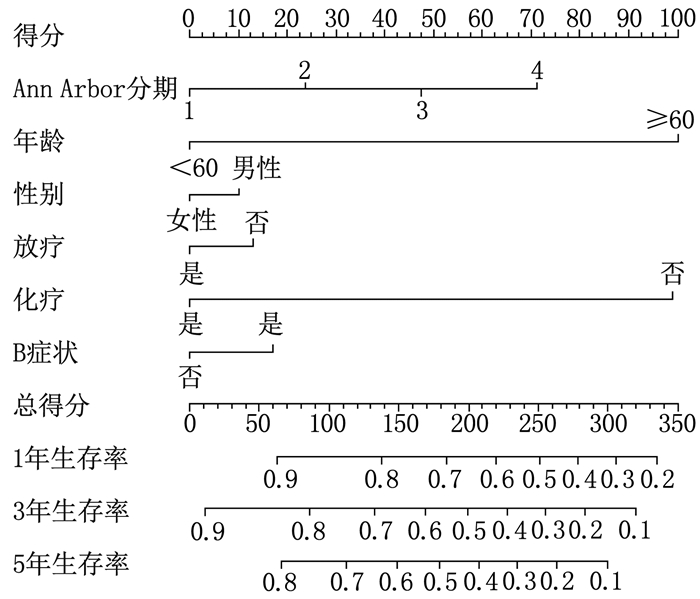
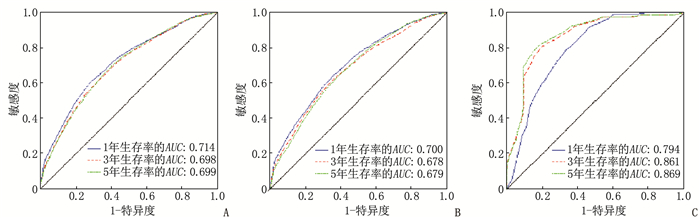
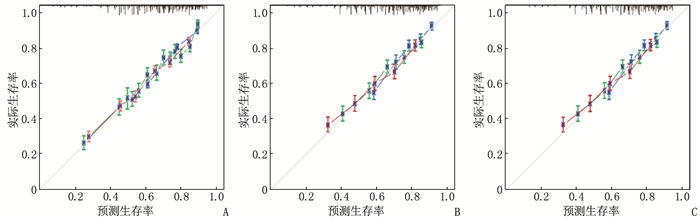
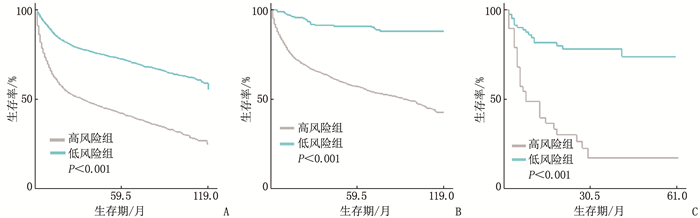
结果多因素Cox回归分析结果显示, 年龄≥ 60岁、男性、Ann Arbor分期高、有B症状、未接受放疗、未接受化疗是影响DLBCL预后的危险因素(P < 0.05), 同时将上述因素纳入列线图模型。SEER训练集、验证集以及外部验证集的C-index分别为0.681、0.669、0.817, SEER训练集、验证集以及外部验证集ROC曲线预测DLBCL患者5年生存率的AUC分别为0.699、0.678、0.869。校准曲线显示列线图预测模型结果与实际结果具有良好的一致性。计算患者的风险评分并将其分为低风险组(< 170分)和高风险组(≥ 170分), 低风险组的5年生存率在SEER训练集、SEER验证集以及外部验证集中均为最高。
结论男性、年龄≥ 60岁、Ann Arbor分期高、未接受放疗、未接受化疗、存在B症状是影响DLBCL患者预后的独立危险因素。基于上述因素建立的列线图预测模型具有良好的预测效能, 可对DLBCL患者预后进行个体化风险评估和预测。
-
关键词:
- 弥漫性大B细胞淋巴瘤 /
- 校准曲线 /
- 美国国家癌症研究所监测、流行病学和最终结果数据库 /
- 列线图 /
- 放疗 /
- 化疗
Abstract:ObjectiveTo analyze the related factors affecting the prognosis of patients with diffuse large B-cell lymphoma (DLBCL), and to establish a Nomogram for prediction of the prognosis of patients with DLBCL.
MethodsThe baseline data of 9 486 patients with DLBCL were extracted from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, and the SEER data were randomly divided into SEER training set (n=6 681) and SEER validation set (n=2 805) according to the ratio of 7 to 3. Univariate and multivariate Cox regression analyses were used to determine the risk factors affecting the prognosis of patients with DLBCL, and on this basis, a Nomogram was established; the discrimination and prediction efficiency of the model were evaluated by consistency index (C-index), area under the curve (AUC) of receiver operating characteristic (ROC) curve and calibration curve. A total of 120 patients with DLBCL in the Department of Hematology and Department of Oncology in authors' hospital from January 2013 to December 2017 were selected as external validation set, and the external validation was performed to verify the Nomogram and analyze its influencing factors.
ResultsMultivariate Cox regression analysis showed that age ≥ 60 years old, male, high stages of Ann Arbor staging, presence of B symptoms, lack of radiotherapy and lack of chemotherapy were the risk factors affecting the prognosis of DLBCL (P < 0.05), and the above factors were included in the Nomogram model. The C-index values of SEER training set, validation set and external validation set were 0.681, 0.669 and 0.817 respectively, and the AUC values of ROC curves of SEER training set, validation set and external validation set for predicting 5-year survival rate of DLBCL patients were 0.699, 0.678 and 0.869 respectively. The calibration curve showed that the result of the Nomogram prediction model was in good consistency with the actual result. Patients were divided into low-risk group (< 170 points) and high-risk group (≥ 170 points) according to the calculated risk scores, and the 5-year survival rate of the low-risk group was the highest in the SEER training set, validation set and external validation set.
ConclusionMale, age ≥ 60 years old, high stages of Ann Arbor staging, lack of radiotherapy, lack of chemotherapy and presence of B symptoms are the independent risk factors affecting the prognosis of patients with DLBCL. The Nomogram prediction model established based on the above factors has good predictive performance, and can be used for personalized risk assessment and prediction of the prognosis of patients with DLBCL.
-
-
表 1 SEER训练集DLBCL患者预后的Cox回归分析
因素 单因素分析 多因素分析 HR(95%CI) P HR(95%CI) P 年龄 < 0.001 < 0.001 < 60岁 1 1 ≥60岁 2.789(2.536~3.066) < 0.001 2.720(2.473~2.992) < 0.001 性别 < 0.001 < 0.001 女 1 1 男 1.259(1.169~1.356) < 0.001 1.336(1.240~1.440) < 0.001 Ann Arbor分期 < 0.001 < 0.001 Ⅰ期 1 1 Ⅱ期 1.107(0.985~1.245) 0.089 1.272(1.129~1.433) < 0.001 Ⅲ期 1.586(1.418~1.775) < 0.001 1.777(1.578~2.000) < 0.001 Ⅳ期 1.850(1.676~2.043) < 0.001 2.036(1.835~2.259) < 0.001 放疗 < 0.001 0.028 是 1 1 否 1.451(1.324~1.590) < 0.001 1.113(1.012~1.223) 0.028 化疗 < 0.001 < 0.001 是 1 1 否 2.519(2.308~2.748) < 0.001 2.677(2.446~2.929) < 0.001 B症状 < 0.001 < 0.001 无 1 1 有 1.182(1.093~1.279) < 0.001 1.171(1.080~1.270) < 0.001 表 2 外部验证集DLBCL患者预后的Cox回归分析
因素 单因素分析 多因素分析 HR(95%CI) P HR(95%CI) P 年龄 0.141 < 60岁 1 — — ≥60岁 1.777(0.827~3.817) 0.141 — — 性别 0.962 女 1 — — 男 1.015(0.561~1.834) 0.962 — — Ann Arbor分期 < 0.001 < 0.001 Ⅰ期 1 1 Ⅱ期 3.03(1.036~8.869) 0.043 2.578(0.876~7.585) 0.085 Ⅲ期 3.806(1.321~10.964) 0.013 3.627(1.259~10.450) 0.017 Ⅳ期 8.285(3.079~22.290) < 0.001 7.216(2.678~19.442) < 0.001 放疗 0.101 是 1 — — 否 2.055(0.869~4.863) 0.101 — — 化疗 < 0.001 < 0.001 是 1 1 否 4.221(2.328~7.653) < 0.001 3.784(2.085~6.868) < 0.001 B症状 0.887 无 1 — — 有 0.940(0.398~2.220) 0.887 — — -
[1] SUKSWAI N, LYAPICHEV K, KHOURY J D, et al. Diffuse large B-cell lymphoma variants: an update[J]. Pathology, 2020, 52(1): 53-67. doi: 10.1016/j.pathol.2019.08.013
[2] LIU Y, BARTA S K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment[J]. Am J Hematol, 2019, 94(5): 604-616. doi: 10.1002/ajh.25460
[3] TWA D D W, MOTTOK A, SAVAGE K J, et al. The pathobiology of primary testicular diffuse large B-cell lymphoma: implications for novel therapies[J]. Blood Rev, 2018, 32(3): 249-255. doi: 10.1016/j.blre.2017.12.001
[4] International non-Hodgkin's lymphoma prognostic factors project. A predictive model for aggressive non-Hodgkin's lymphoma[J]. N Engl J Med, 1993, 329(14): 987-994. doi: 10.1056/NEJM199309303291402
[5] PAN Y X, CHEN J C, FANG A P, et al. A nomogram predicting the recurrence of hepatocellular carcinoma in patients after laparoscopic hepatectomy[J]. Cancer Commun, 2019, 39(1): 55.
[6] RUPPERT A S, DIXON J G, SALLES G, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI[J]. Blood, 2020, 135(23): 2041-2048. doi: 10.1182/blood.2019002729
[7] CHIAPPELLA A, CASTELLINO A, NICOLOSI M, et al. Diffuse Large B-cell Lymphoma in the elderly: standard treatment and new perspectives[J]. Expert Rev Hematol, 2017, 10(4): 289-297. doi: 10.1080/17474086.2017.1305264
[8] SHAH B K, BISTA A, SHAFII B. Disparities in receipt of radiotherapy and survival by age, sex and ethnicity among patients with stage Ⅰ diffuse large B-cell lymphoma[J]. Leuk Lymphoma, 2015, 56(4): 983-986. doi: 10.3109/10428194.2014.940583
[9] 赵志强, 魏俏俏, 范双龙, 等. 老年弥漫大B细胞淋巴瘤患者预后影响因素分析[J]. 肿瘤研究与临床, 2022, 34(2): 128-131. https://cdmd.cnki.com.cn/Article/CDMD-11810-1022744987.htm [10] 翟淑丹, 朱磊, 张利卜, 等. 18F-FDG PET/CT对弥漫大B细胞淋巴瘤预后的预测价值[J]. 癌症, 2021, 40(9): 404-411. https://www.cnki.com.cn/Article/CJFDTOTAL-AIZH202112005.htm [11] 谭晓虹, 孙洁, 冯荣浩, 等. 153例弥漫性大B细胞淋巴瘤的临床病理特征及预后相关因素分析[J]. 肿瘤预防与治疗, 2020, 33(8): 645-650. https://www.cnki.com.cn/Article/CJFDTOTAL-SCZF202008003.htm [12] 田晨, 任洪涛, 姜成毅, 等. 初治弥漫大B细胞淋巴瘤患者临床、基因特征及预后的影响因素[J]. 中国老年学杂志, 2020, 40(22): 4736-4740. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLXZ202022012.htm [13] HAN Y, QIN Y, HE X H, et al. Retrospective analysis of the clinical features and prognostic factors of 370 patients with advanced-stage diffuse large B-cell lymphoma[J]. Chin J Oncol, 2018, 40(6): 456-461.
[14] LIU P P, WANG K F, JIN J T, et al. Role of radiation therapy in primary breast diffuse large B-cell lymphoma in the Rituximab era: a SEER database analysis[J]. Cancer Med, 2018, 7(5): 1845-1851. doi: 10.1002/cam4.1457
[15] WU J Q, SONG Y P, SU L P, et al. Rituximab plus chemotherapy as first-line treatment in Chinese patients with diffuse large B-cell lymphoma in routine practice: a prospective, multicentre, non-interventional study[J]. BMC Cancer, 2016, 16: 537. doi: 10.1186/s12885-016-2523-7
[16] LIN J Y, ZHENG Y B, HE H M, et al. Clinicopathological Features and Prognostic Factors of DLBCL[J]. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 2018, 26(3): 779-783.
[17] 陈雅姝, 刘敏, 王俊. 利妥昔单抗治疗弥漫大B细胞淋巴瘤的疗效及影响患者预后多因素分析[J]. 实用癌症杂志, 2020, 35(6): 1031-1034. https://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ202006043.htm [18] 汪玉芳, 柯金勇, 柯善栋. 原发性胃弥漫大B细胞淋巴瘤患者的生存情况及其影响因素分析[J]. 癌症进展, 2018, 16(8): 1020-1023. https://www.cnki.com.cn/Article/CJFDTOTAL-AZJZ201808027.htm [19] ZUO Z C, ZHANG G C, SONG P, et al. Survival nomogram for stage IB non-small-cell lung cancer patients, based on the SEER database and an external validation cohort[J]. Ann Surg Oncol, 2021, 28(7): 3941-3950.
[20] HAN Y, YANG J L, LIU P, et al. Prognostic nomogram for overall survival in patients with diffuse large B-cell lymphoma[J]. Oncologist, 2019, 24(11): e1251-e1261.
[21] WANG J, ZHOU M, ZHOU R F, et al. Nomogram for predicting the overall survival of adult patients with primary gastrointestinal diffuse large B cell lymphoma: a SEER-based study[J]. Front Oncol, 2020, 10: 1093.
[22] RAMSPEK C L, JAGER K J, DEKKER F W, et al. External validation of prognostic models: what, why, how, when and where[J]. Clin Kidney J, 2021, 14(1): 49-58.
-
期刊类型引用(12)
1. 杨林,许彬,吴娟,毕立清,冒萧萧,镇坷,侯慧,徐修鹏. 可视化鼻肠管置入术在神经外科重症患者中的应用效果观察. 实用临床医药杂志. 2023(22): 13-16+23 .  本站查看
本站查看
2. 郭文超,秦寒枝,滕娇,金魁,孙建,王忠丽,蒋燕. 成人重型颅脑损伤患者肠内营养支持的最佳证据总结. 中国全科医学. 2022(15): 1825-1832 .  百度学术
百度学术
3. 孙秋红,习文艳. 神经外科重症患者营养风险发生率及营养支持的效果分析. 中国药物与临床. 2021(18): 3190-3192 .  百度学术
百度学术
4. 郭晨. 神经外科重症患者营养风险发生率及营养支持的效果分析. 首都食品与医药. 2020(06): 26-27 .  百度学术
百度学术
5. 段娟,李丹,程琳,夏璇,施雁. 老年脑出血患者营养不良筛查及影响因素的研究进展. 西北国防医学杂志. 2020(03): 186-192 .  百度学术
百度学术
6. 吴昌徽,程琼,张婷. 神经危重症患者营养风险评分及营养评估研究. 当代医学. 2020(11): 28-30 .  百度学术
百度学术
7. 邓开勇,刘福,贺美波,周超. 宽中健脾汤联合肠内营养在神经危重症患者治疗中的临床应用. 四川中医. 2020(09): 89-93 .  百度学术
百度学术
8. 梁敏. 幽门后喂养在神经外科高龄重症患者中应用效果. 按摩与康复医学. 2019(15): 27-28 .  百度学术
百度学术
9. 刘月雯,邓瑛瑛,刘丹,黄笑,邱炳辉. 腹部按摩联合超声治疗仪对神经外科重症患者胃肠功能和营养状况的影响. 中国医药导报. 2019(26): 78-81 .  百度学术
百度学术
10. 陈芹,卢筱蔚,杨晓洋. 营养管理模式对神经外科重症患者营养摄入的影响. 实用临床护理学电子杂志. 2018(03): 66+69 .  百度学术
百度学术
11. 刘新平,赵新英,丁梅,房宁宁,欧阳修河. NRS2002评分预测ICU成人重症患者预后的临床研究. 中华全科医学. 2018(10): 1629-1631+1666 .  百度学术
百度学术
12. 赵欣,魏园,于媛媛,贾蓓,郭世文. 电磁导航系统引导定位在床旁空肠营养管留置中的应用研究. 中国医学装备. 2018(08): 36-39 .  百度学术
百度学术
其他类型引用(3)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
