Value of pre-treatment pan-immune inflammation score in predicting prognosis of esophageal cancer patients with postoperative adjuvant radiotherapy
-
摘要:目的
探讨接受术后辅助放疗的食管鳞状细胞癌患者治疗前泛免疫炎症值(PIV)与临床病理特征的相关性,并联合T分期评估其在食管鳞癌患者预后中的价值。
方法回顾性收集2019年1月—2023年1月在扬州大学附属医院放射肿瘤科行术后辅助放疗的食管鳞癌患者85例的临床资料。绘制受试者工作特征(ROC)曲线获取PIV和其他免疫炎症生物标志物的最佳临界值,依据ROC曲线及决策曲线分析(DCA)比较PIV和其他免疫炎症生物标志物的曲线下面积(AUC)及临床适用性; 根据最佳临界值将患者分为PIV高水平组和PIV低水平组,评估PIV水平与食管鳞癌临床病理特征的相关性。生存分析采用Kaplan-Meier法,多因素分析采用Cox比例风险模型,并通过递归分区分析(RPA)建立一个结合PIV和T分期的风险分层模型。
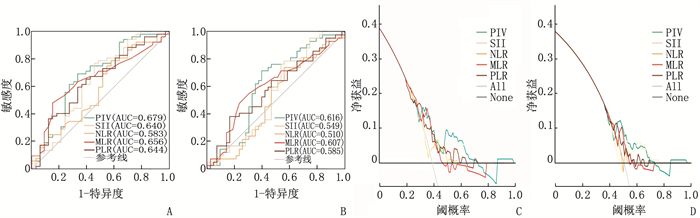
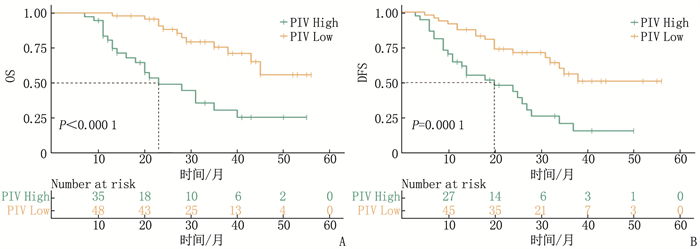
结果根据ROC曲线确定治疗前PIV最佳临界值为187.22, PIV的ROC曲线的AUC(0.679)大于其他4项[全身免疫炎症指数(SII)、血小板与淋巴细胞比值(PLR)、单核细胞与淋巴细胞比值(MLR)、中性粒细胞与淋巴细胞比值(NLR)]免疫炎症生物标志物(0.640、0.583、0.656、0.644)。将85例患者分为PIV低水平组(< 187.22)48例和PIV高水平组(≥187.22)37例, PIV的水平高低与肿瘤直径相关(P < 0.05)。PIV低水平组3年总生存期(OS)(75.6%与30.6%, P < 0.001)和3年无病生存期(DFS)(56.1%与21.0%, P < 0.001)高于PIV高水平组; 肿瘤直径、T分期和PIV是食管鳞癌患者OS的独立影响因素(P < 0.05), T分期和PIV是食管鳞癌患者DFS的独立影响因素(P < 0.05)。采用基于T分期和PIV的RPA分层模型建立了一个包含3个风险组的新分期,与单独的T分期或PIV相比,基于RPA生成的模型可进一步提高对预后的预测价值。
结论治疗前PIV有助于预测术后辅助放疗食管鳞癌患者预后, PIV联合T分期可提高预测价值。
Abstract:ObjectiveTo investigate the correlation between pre-treatment pan-immune inflammation value (PIV) and clinicopathological features in esophageal squamous cell carcinoma (ESCC) patients with postoperative adjuvant radiotherapy and evaluate its value in prognosis assessment combined with T stage.
MethodsA retrospective analysis was conducted on data of 85 ESCC patients with postoperative adjuvant radiotherapy in the Department of Radiation Oncology of the Affiliated Hospital of Yangzhou University from January 2019 to January 2023. The receiver operating characteristic (ROC) curve was drew to obtain the optimal cut-off value of PIV and other immune-inflammatory biomarkers. The area under the curve (AUC) and clinical applicability of PIV and other immune-inflammatory biomarkers were compared based on the ROC curve and decision curve analysis (DCA). According to the optimal cut-off value, patients were divided into high PIV group and low PIV group, and the correlation between PIV level and clinicopathological features of ESCC was evaluated. Kaplan-Meier method was used for survival analysis, the Cox proportional hazards model was used for multivariate analysis, and a risk stratification model combining PIV and T stage was established by recursive partitioning analysis (RPA).
ResultsThe optimal cut-off value of pre-treatment PIV was determined as 187.22 based on the ROC curve. The AUC of PIV was 0.679, which was greater than 0.640, 0.583, 0.656 and 0.644 of the other four immune-inflammatory biomarkers such as the systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and neutrophil-to-lymphocyte ratio (NLR). The 85 patients were divided into low PIV group (< 187.22, n=48) and high PIV group (≥187.22, n=37). The level of PIV was significantly correlated with tumor diameter (P < 0.05). The 3-year overall survival (OS) (75.6% versus 30.6%, P < 0.001) and 3-year disease-free survival (DFS) (56.1% versus 21.0%, P < 0.001) were significantly higher in the low PIV group than the high PIV group. Tumor diameter, T stage and PIV were independent factors affecting OS in ESCC patients (P < 0.05), and T stage and PIV were independent factors affecting DFS in ESCC patients (P < 0.05). A new staging system with three risk groups was established by the RPA stratification model based on T stage and PIV, which further improved the predictive value of prognosis compared with T stage or PIV alone.
ConclusionPre-treatment PIV is helpful in predicting the prognosis of ESCC patients with postoperative adjuvant radiotherapy, and the combination of PIV and T stage can improve the predictive value.
-
在亚洲地区,约90%的食管癌病例通过病理学检查被确诊为鳞状细胞癌[1-2]。近年来,食管癌的治疗趋向于多学科诊疗模式,但鉴于肿瘤的转移和复发,患者的5年生存率仍不佳[3-5]。免疫炎症生物标志物如淋巴细胞、血小板、单核细胞和中性粒细胞能反映患者的免疫和炎症状态,在预测肿瘤预后方面具有关键作用[6-7]。此外,一些综合性的免疫炎症生物标志物如全身免疫炎症指数(SII)、血小板与淋巴细胞比值(PLR)、单核细胞与淋巴细胞比值(MLR)、中性粒细胞与淋巴细胞比值(NLR)也具有一定的预后预测价值[8-10]。泛免疫炎症值(PIV)是一种包含中性粒细胞、血小板、单核细胞和淋巴细胞共4个参数的复合免疫炎症生物标志物,其在预测结直肠癌预后方面优于其他免疫炎症生物标志物[11-12]。PIV在前列腺癌、黑色素瘤、乳腺癌、肺癌等多种肿瘤[13-17]中的预后价值也得到了证实。本研究探讨治疗前PIV对食管鳞癌患者术后辅助放疗预后的评估价值,现报告如下。
1. 资料与方法
1.1 一般资料
回顾性收集2019年1月—2023年1月在扬州大学附属医院放射肿瘤科行辅助放射治疗的食管鳞癌患者的临床资料。纳入标准: ①患者治疗前实验室检查数据完整; ②肿瘤术后病理明确为食管鳞癌; ③肿瘤手术为R0切除; ④术后2个月内开始行术后辅助放疗并按计划完成者。排除标准: ①手术前存在严重感染或免疫抑制者; ②既往接受过手术外的其他抗肿瘤治疗者; ③有严重的基础疾病而影响肿瘤治疗者; ④同时患有其他恶性肿瘤者。本研究经过扬州大学附属医院伦理委员会的批准[文件号: 2023-YKL04-(课05)], 所有患者知情并签署同意书。
1.2 放疗靶区及计划方案
85例食管鳞癌患者均接受三维适形调强放射治疗(IMRT), 根据胸部CT定位图像,结合食管钡餐造影和(或)PET(CT)等影像学结果勾画靶区。临床靶区(CTV)包括原发肿瘤部位对应淋巴结引流区,颈段及胸上段CTV同时包括手术吻合口,计划靶区(PTV)为CTV均匀外扩0.5~1.0 cm。95%PTV处方剂量为50~56 Gy, 单次剂量为1.8~2.0 Gy, 放疗每日1次,每周5次。正常组织的放疗耐受剂量限制: 双肺受到5 Gy辐射剂量的体积(V5)≤60%, V20≤30%, V30≤20%; 心脏平均照射剂量 < 30 Gy, V25≤50%, V40≤30%, 脊髓最高照射剂量 < 45 Gy。
1.3 免疫炎症生物标志物
依据患者术前1周内进行的血常规检查进行计算。PIV=(血小板计数×中性粒细胞计数×单核细胞计数)/淋巴细胞计数, SII=(血小板计数×中性粒细胞计数)/淋巴细胞计数, NLR=中性粒细胞计数/淋巴细胞计数, MLR=单核细胞计数/淋巴细胞计数, PLR=血小板计数/淋巴细胞计数。绘制受试者工作特征(ROC)曲线获取PIV、SII、NLR、MLR及PLR的最佳临界值,根据最佳临界值将患者分为高水平组与低水平组,并根据ROC曲线的曲线下面积(AUC)评估其预测效能。
1.4 随访
放疗结束后对所有患者进行电话、门诊或再次入院随访,放疗后2年内为每3个月随访1次, 2年后为每6个月随访1次。随访截至2023年12月31日。总生存期(OS)是指从患者确诊至任何原因引起死亡或末次随访的时间,失访患者为末次随访时间; 无病生存期(DFS)是指患者从确诊到第1次肿瘤复发或转移或因任何原因导致死亡的时间,失访患者为末次随访时间。
1.5 统计学分析
采用SPSS 25.0和R4.2.3进行数据分析。对连续变量进行Student′t检验或Mann-Whitney U检验,对分类变量分别进行卡方检验或Fisher精确检验。根据ROC曲线比较PIV和其他免疫炎症生物标志物的AUC。根据决策曲线分析(DCA)比较和评估PIV和其他免疫炎症生物标志物的临床适用性。通过Kaplan-Meier生存分析比较生存差异。单因素分析采用Log-rank卡方检验,将单因素分析中P < 0.05的因素纳入Cox回归模型进行多因素分析。通过递归分区分析(RPA)建立结合PIV和T分期的风险分层模型以进行预测和分层,采用ROC曲线和DCA评估基于RPA的分层模型的预后性能。P < 0.05为差异有统计学意义。
2. 结果
2.1 纳入患者的特征
根据排除和纳入标准,共纳入食管癌患者85例,年龄47~81岁,平均(67.12±6.65)岁,男58例,女27例; 根据2017年美国肿瘤联合会(AJCC)指南(第8版)[18]进行食管鳞状细胞癌的手术后TNM分期,其中Ⅱ期48例, Ⅲ期37例; 接受同期化疗38例,其中TP方案(顺铂+紫杉醇)12例, FP方案(顺铂+氟尿嘧啶)5例, S1方案(替吉奥)21例,另有47例患者仅接受辅助放射治疗; 随访7~56个月,中位随访时间为27.75个月; 复发43例(50.59%), 死亡33例(38.82%)。86例患者临床病理特征和血液学免疫炎症生物标志物见表 1。
表 1 食管癌患者的临床病理特征及血液学指标(n=85)(x±s)[n(%)]指标 分类 数据 指标 分类 数据 性别 男 58(68.23) N分期 N0期 46(54.12) 女 27(31.77) N1期 28(32.94) 吸烟史 有 43(50.59) N2期 11(12.94) 无 42(49.41) pTNM分期 Ⅱ期 48(56.47) 饮酒史 有 34(40.00) Ⅲ期 37(43.53) 无 51(60.00) 手术方式 胸腔镜 75(88.23) 肿瘤位置 胸上段 5(5.89) 开胸手术 10(11.77) 胸中段 35(41.18) 同期化疗 有 38(44.70) 胸下段 45(52.93) 无 47(55.30) 分化程度 高分化 4(4.70) 化疗方案 未同期化疗 47(55.29) 中分化 54(63.53) 紫杉醇联合顺铂方案 12(14.12) 低分化 27(31.77) 氟尿嘧啶联合顺铂方案 5(5.88) 脉管侵犯 有 23(27.06) 替吉奥单药方案 21(24.71) 无 62(72.94) 肿瘤直径/cm 3.64±1.47 神经侵犯 有 12(14.12) PIV 245.19±217.93 无 73(85.88) SII 617.71±366.64 T分期 T1期 3(3.53) NLR 3.22±1.34 T2期 32(37.65) MLR 0.30±0.11 T3期 50(58.82) PLR 150.83±59.59 年龄/岁 67.10±6.60 pTNM分期: 手术后TNM分期; PIV: 泛免疫炎症值; SII: 全身免疫炎症指数; NLR: 中性粒细胞与淋巴细胞比值; MLR: 单核细胞与淋巴细胞比值; PLR: 血小板与淋巴细胞比值。 2.2 免疫炎症生物标志物的预后价值
对PIV及其他经典免疫炎症生物标志物(SII、NLR、MLR及PLR)进行ROC曲线和DCA分析。根据总生存期绘制ROC曲线, PIV、SII、NLR、MLR及PLR的最佳临界值分别为187.22、645.48、3.77、0.35、149.27。与其他经典免疫炎症生物标志物相比, PIV的AUC最大(AUCOS=0.679, AUCDFS=0.616), 表明PIV预测预后的价值高于其他免疫炎症生物标志物; 此外,与其他免疫炎症生物标志物相比,DCA显示PIV预测OS和DFS的价值高于其他免疫炎症生物标志物。见图 1。
2.3 PIV与患者临床病理特征的关系
根据治疗前PIV的最佳临界值将患者分为PIV高水平组37例和PIV低水平组48例,分析不同水平PIV与食管鳞癌术后辅助放疗患者临床病理特征的关系。结果发现2组肿瘤直径的差异有统计学意义(P < 0.05), 2组性别、年龄、吸烟史、饮酒史、肿瘤位置、分化程度、T分期、淋巴结转移、pTNM分期、手术方式及化疗方案、神经及脉管侵犯的差异无统计学意义(P>0.05), 见表 2。
表 2 食管癌患者PIV与临床病理特征的关系[n(%)]指标 分类 总例数(n=85) PIV高水平组(n=37) PIV低水平组(n=48) P 性别 女 27(31.8) 11(29.7) 16(33.3) 0.905 男 58(68.2) 26(70.3) 32(66.7) 年龄 < 65岁 21(24.7) 11(29.7) 10(20.8) 0.491 ≥65岁 64(75.3) 26(70.3) 38(79.2) 吸烟史 无 42(49.4) 17(45.9) 25(52.1) 0.732 有 43(50.6) 20(54.1) 23(47.9) 饮酒史 无 51(60.0) 23(62.2) 28(58.3) 0.893 有 34(40.0) 14(37.8) 20(41.7) 肿瘤位置 胸中上段 40(47.1) 16(43.2) 24(50.0) 0.689 胸下段 45(52.9) 21(56.8) 24(50.0) 分化程度 高分化 4(4.7) 3(8.1) 1(2.1) 0.476 中分化 54(63.5) 23(62.2) 31(64.6) 低分化 27(31.8) 11(29.7) 16(33.3) 脉管侵犯 无 62(72.9) 27(73.0) 35(72.9) 0.935 有 23(27.1) 10(27.0) 13(27.1) 神经侵犯 有 12(14.1) 5(13.5) 7(14.6) 0.921 无 73(85.9) 32(86.5) 41(85.4) 肿瘤直径 < 3.25 cm 42(49.4) 12(32.4) 30(62.5) 0.011 ≥3.25 cm 43(50.6) 25(67.6) 18(37.5) T分期 T1~T2期 35(41.2) 11(29.7) 24(50.0) 0.097 T3期 50(58.8) 26(70.3) 24(50.0) N分期 N0期 46(54.1) 20(54.1) 26(54.2) 0.931 N1~N2期 39(45.9) 17(45.9) 22(45.8) pTNM分期 Ⅱ期 48(56.5) 20(54.1) 28(58.3) 0.927 Ⅲ期 37(43.5) 17(45.9) 20(41.7) 手术方式 胸腔镜 75(88.2) 31(83.8) 44(91.7) 0.320 开胸手术 10(11.8) 6(16.2) 4(8.3) 同期化疗 无 47(55.3) 17(45.9) 30(62.5) 0.193 有 38(44.7) 20(54.1) 18(37.5) 化疗方案 未同期化疗 47(55.3) 17(45.9) 30(62.5) 0.373 紫杉醇联合顺铂方案 11(12.9) 6(16.2) 5(10.4) 氟尿嘧啶联合顺铂方案 6(7.1) 3(8.1) 3(6.2) 替吉奥单药方案 21(24.7) 11(29.8) 10(20.9) PIV: 泛免疫炎症值; pTNM分期: 手术后TNM分期。 2.4 生存分析及预后影响因素分析
对85例食管鳞癌术后患者进行7~56个月的随访,中位随访时间26个月,其中33例患者死亡(38.82%), 52例患者存活(61.18%)。PIV低水平组3年OS(75.6%与30.6%, P < 0.001)、3年DFS(56.1%与21.0%, P < 0.001)高于PIV高水平组,见图 2。单因素分析显示肿瘤直径、T分期、PIV与食管鳞癌患者OS及DFS显著相关(P < 0.05), 见表 3。将单因素分析中有统计学意义的因素进行多因素回归分析,结果显示肿瘤直径、T分期和PIV为患者OS的独立影响因素(P < 0.05), 而T分期和PIV为患者DFS的独立影响因素(P < 0.05); 与PIV高水平组相比,PIV低水平组的复发和死亡风险分别减少了0.398和0.321倍,见表 4。
表 3 食管癌术后辅助放疗患者总生存率和无病生存率的单因素分析因素 分类 总生存率HR(95%CI) P 无病生存率HR(95%CI) P 性别 女 参照 — 参照 — 男 2.238(0.923~5.427) 0.075 1.672(0.823~3.394) 0.155 年龄 < 65岁 参照 — 参照 — ≥65岁 1.075(0.484~2.384) 0.859 0.908(0.465~1.772) 0.777 吸烟史 无 参照 — 参照 — 有 0.993(0.498~1.979) 0.984 1.084(0.593~1.984) 0.793 饮酒史 无 参照 — 参照 — 有 0.929(0.457~1.889) 0.838 1.119(0.610~2.053) 0.717 肿瘤位置 胸中上段 参照 — 参照 — 胸下段 1.268(0.635~2.531) 0.501 1.071(0.586~1.957) 0.825 分化程度 高分化 参照 — 参照 — 中分化 0.329(0.075~1.443) 0.141 0.299(0.088~1.025) 0.055 低分化 0.552(0.122~2.505) 0.441 0.455(0.129~1.614) 0.223 脉管侵犯 无 参照 — 参照 — 有 1.044(0.496~2.201) 0.909 1.278(0.674~2.420) 0.452 神经侵犯 无 参照 — 参照 — 有 0.848(0.254~2.824) 0.788 0.959(0.375~2.457) 0.931 肿瘤直径 < 3.25 cm 参照 — 参照 — ≥3.25 cm 4.362(1.958~9.717) < 0.001 2.265(1.217~4.214) 0.010 T分期 T1~T2期 参照 — 参照 — T3期 5.036(2.063~12.293) < 0.001 5.711(2.653~12.294) < 0.001 N分期 N0期 参照 — 参照 — N1~N2期 0.817(0.406~1.643) 0.57 0.841(0.459~1.542) 0.576 pTNM分期 Ⅱ期 参照 — 参照 — Ⅲ期 0.861(0.428~1.732) 0.674 0.893(0.487~1.638) 0.715 手术方式 胸腔镜 参照 — 参照 — 开胸手术 1.589(0.655~3.855) 0.306 1.086(0.458~2.576) 0.851 同期化疗 无 参照 — 参照 — 有 1.328(0.664~2.657) 0.423 1.557(0.850~2.852) 0.151 化疗方案 未同期化疗 参照 — 参照 — 紫杉醇联合顺铂方案 2.109(0.827~5.377) 0.118 1.601(0.680~3.772) 0.282 氟尿嘧啶联合顺铂方案 0.983(0.226~4.271) 0.982 0.730(0.171~3.106) 0.670 替吉奥单药方案 1.302(0.538~3.151) 0.559 1.914(0.934~3.921) 0.076 PIV 高 参照 — 参照 — 低 0.244(0.119~0.500) < 0.001 0.340(0.184~0.627) 0.001 HR: 风险比; CI: 置信区间; pTNM分期: 手术后TNM分期; PIV: 泛免疫炎症值。 表 4 食管癌术后辅助放疗患者总生存率和无病生存率的多因素分析因素 分类 总生存率HR(95%CI) P 无病生存率HR(95%CI) P 肿瘤直径 < 3.25 cm 参照 — 参照 — ≥3.25 cm 2.554(1.096~5.953) 0.030 1.188(0.614~2.298) 0.609 T分期 T1~T2期 参照 — 参照 — T3期 3.389(1.344~8.542) 0.010 5.059(2.225~11.505) < 0.001 PIV 高 参照 — 参照 — 低 0.321(0.154~0.669) 0.002 0.398(0.212~0.750) 0.004 HR: 风险比; CI: 置信区间; PIV: 泛免疫炎症值。 2.5 基于PIV和pT分期的风险分层模型构建
采用基于PIV和pT分期的RPA分层模型进行新的分期; RPA模型将85例食管鳞癌患者分为RPA Ⅰ(pT分期Ⅰ~Ⅱ期和低水平PIV, n=24)、RPA Ⅱ(pT分期Ⅰ~Ⅱ期和高水平PIV或pT分期Ⅲ期和低水平PIV, n=35)和RPA Ⅲ(pT分期Ⅲ期和高水平PIV, n=26); 与RPA Ⅰ相比, RPA Ⅱ和RPA Ⅲ的复发风险提升了5.447倍和15.104倍,死亡风险分别提升了6.521倍和20.877倍。根据DFS和OS绘制的生存曲线, RPA模型也具有更好的分层效果; RPA模型的预测性能也与T分期及PIV进行了比较,从ROC曲线来看,基于RPA分层模型的DFS和OS预测精度高于单独的T分期或PIV; 同样, DCA曲线也支持RPA分层模型有更高的预测效能。见图 3。
3. 讨论
随着医疗技术的不断进步,肿瘤治疗方式趋向多样化与精准化。治疗前对肿瘤患者预后的准确预测尤为关键。本研究结果显示,原发肿瘤负荷较高的患者PIV基线值较高,这与文献[19]中关于肿瘤相关免疫及炎症状态与肿瘤侵袭性及转移能力关系的描述相吻合。值得注意的是,在众多成熟且常用的免疫炎症生物标志物中, PIV在ROC和DCA曲线中展现出的预测效能更优。PIV是基于免疫炎症的食管鳞状细胞癌预后分层的优选指标。
PIV于2020年首次提出,相较于其他传统免疫炎症生物标志物, PIV能更显著地影响转移性结直肠癌患者的预后[12]。PIV在多种恶性肿瘤中的预后价值得到了广泛认可[20-22], 尽管不同研究采用的标准各异, PIV的预测价值已在多项荟萃分析[23-24]中得到确认,包括针对结直肠癌的6项研究以及涵盖15项各种肿瘤研究的荟萃分析。此外, PIV在多种接受免疫治疗的恶性肿瘤中的预后作用也得到了证实[13, 15]。研究[25]指出, PIV可预测接受根治性切除的食管鳞癌患者的预后,但该研究中治疗方法不一,包括仅接受手术和接受辅助治疗的患者,这可能导致PIV最佳临界值存在差异。本研究中,PIV与肿瘤直径呈正相关,较长的肿瘤可能导致PIV较高的患者处于促肿瘤的免疫炎症状态。本研究采用结合T分期与PIV的RPA模型,对食管鳞癌患者进行了新的分层。RPA分层模型将85例患者分为3个风险等级,展现了更佳的分层效果,相较于T分期和PIV,其生存预测的准确性得到了进一步提升。
除了肿瘤自身的影响,免疫和炎症状态也对肿瘤预后有着重要影响[26]。免疫炎症生物标志物在预测肿瘤预后方面具有重要作用,可以反映宿主免疫和炎症状态的平衡[7]。PIV作为一种综合性免疫炎症生物标志物,其反映了血小板通过与循环中的肿瘤细胞连接产生血栓,帮助肿瘤细胞逃避免疫系统的攻击; 激活的血小板产生多种生理活性细胞因子,促进肿瘤的迁移和侵袭[27]。中性粒细胞具有调节肿瘤微环境的能力,可促进肿瘤血管生成、增殖和迁移[28]。肿瘤相关巨噬细胞(TAMs)是激活的单核细胞,通过分泌多种细胞因子促进肿瘤细胞的血管生成、侵袭和转移[29]。淋巴细胞作为衡量免疫功能水平的指标,参与肿瘤微环境的免疫调节,建立对肿瘤细胞的免疫反应[30]。当PIV水平升高时,患者的抗肿瘤炎症反应和免疫功能之间的平衡被打破,推动疾病向着促进肿瘤生成和转移的方向发展,最终导致患者预后不佳。同时,这也提示在治疗前可以根据患者的PIV水平予以抗炎、提高免疫力等对症干预治疗以改善患者预后。
本研究存在的局限性: 首先,本研究作为单一中心的回顾性研究,存在一定的选择偏移; 其次,由于PIV是从外周血中获得,可能会受到多种条件的影响; 再次,本研究的随访时间较短,长期疗效尚需确认; 最后,本研究未能分析PIV在治疗后的数据,结合PIV在术后辅助放疗后的变化或可更准确地预测疗效和预后。目前的研究结果还需在多中心、大样本量的前瞻性研究中进一步验证。
综上所述,对于接受术后辅助放疗的食管鳞癌患者,治疗前PIV是一种新型的、敏感的、有效的预后预测指标。RPA分层模型可以有效帮助临床医生更精准地预测预后并提供个性化的治疗。
-
表 1 食管癌患者的临床病理特征及血液学指标(n=85)(x±s)[n(%)]
指标 分类 数据 指标 分类 数据 性别 男 58(68.23) N分期 N0期 46(54.12) 女 27(31.77) N1期 28(32.94) 吸烟史 有 43(50.59) N2期 11(12.94) 无 42(49.41) pTNM分期 Ⅱ期 48(56.47) 饮酒史 有 34(40.00) Ⅲ期 37(43.53) 无 51(60.00) 手术方式 胸腔镜 75(88.23) 肿瘤位置 胸上段 5(5.89) 开胸手术 10(11.77) 胸中段 35(41.18) 同期化疗 有 38(44.70) 胸下段 45(52.93) 无 47(55.30) 分化程度 高分化 4(4.70) 化疗方案 未同期化疗 47(55.29) 中分化 54(63.53) 紫杉醇联合顺铂方案 12(14.12) 低分化 27(31.77) 氟尿嘧啶联合顺铂方案 5(5.88) 脉管侵犯 有 23(27.06) 替吉奥单药方案 21(24.71) 无 62(72.94) 肿瘤直径/cm 3.64±1.47 神经侵犯 有 12(14.12) PIV 245.19±217.93 无 73(85.88) SII 617.71±366.64 T分期 T1期 3(3.53) NLR 3.22±1.34 T2期 32(37.65) MLR 0.30±0.11 T3期 50(58.82) PLR 150.83±59.59 年龄/岁 67.10±6.60 pTNM分期: 手术后TNM分期; PIV: 泛免疫炎症值; SII: 全身免疫炎症指数; NLR: 中性粒细胞与淋巴细胞比值; MLR: 单核细胞与淋巴细胞比值; PLR: 血小板与淋巴细胞比值。 表 2 食管癌患者PIV与临床病理特征的关系[n(%)]
指标 分类 总例数(n=85) PIV高水平组(n=37) PIV低水平组(n=48) P 性别 女 27(31.8) 11(29.7) 16(33.3) 0.905 男 58(68.2) 26(70.3) 32(66.7) 年龄 < 65岁 21(24.7) 11(29.7) 10(20.8) 0.491 ≥65岁 64(75.3) 26(70.3) 38(79.2) 吸烟史 无 42(49.4) 17(45.9) 25(52.1) 0.732 有 43(50.6) 20(54.1) 23(47.9) 饮酒史 无 51(60.0) 23(62.2) 28(58.3) 0.893 有 34(40.0) 14(37.8) 20(41.7) 肿瘤位置 胸中上段 40(47.1) 16(43.2) 24(50.0) 0.689 胸下段 45(52.9) 21(56.8) 24(50.0) 分化程度 高分化 4(4.7) 3(8.1) 1(2.1) 0.476 中分化 54(63.5) 23(62.2) 31(64.6) 低分化 27(31.8) 11(29.7) 16(33.3) 脉管侵犯 无 62(72.9) 27(73.0) 35(72.9) 0.935 有 23(27.1) 10(27.0) 13(27.1) 神经侵犯 有 12(14.1) 5(13.5) 7(14.6) 0.921 无 73(85.9) 32(86.5) 41(85.4) 肿瘤直径 < 3.25 cm 42(49.4) 12(32.4) 30(62.5) 0.011 ≥3.25 cm 43(50.6) 25(67.6) 18(37.5) T分期 T1~T2期 35(41.2) 11(29.7) 24(50.0) 0.097 T3期 50(58.8) 26(70.3) 24(50.0) N分期 N0期 46(54.1) 20(54.1) 26(54.2) 0.931 N1~N2期 39(45.9) 17(45.9) 22(45.8) pTNM分期 Ⅱ期 48(56.5) 20(54.1) 28(58.3) 0.927 Ⅲ期 37(43.5) 17(45.9) 20(41.7) 手术方式 胸腔镜 75(88.2) 31(83.8) 44(91.7) 0.320 开胸手术 10(11.8) 6(16.2) 4(8.3) 同期化疗 无 47(55.3) 17(45.9) 30(62.5) 0.193 有 38(44.7) 20(54.1) 18(37.5) 化疗方案 未同期化疗 47(55.3) 17(45.9) 30(62.5) 0.373 紫杉醇联合顺铂方案 11(12.9) 6(16.2) 5(10.4) 氟尿嘧啶联合顺铂方案 6(7.1) 3(8.1) 3(6.2) 替吉奥单药方案 21(24.7) 11(29.8) 10(20.9) PIV: 泛免疫炎症值; pTNM分期: 手术后TNM分期。 表 3 食管癌术后辅助放疗患者总生存率和无病生存率的单因素分析
因素 分类 总生存率HR(95%CI) P 无病生存率HR(95%CI) P 性别 女 参照 — 参照 — 男 2.238(0.923~5.427) 0.075 1.672(0.823~3.394) 0.155 年龄 < 65岁 参照 — 参照 — ≥65岁 1.075(0.484~2.384) 0.859 0.908(0.465~1.772) 0.777 吸烟史 无 参照 — 参照 — 有 0.993(0.498~1.979) 0.984 1.084(0.593~1.984) 0.793 饮酒史 无 参照 — 参照 — 有 0.929(0.457~1.889) 0.838 1.119(0.610~2.053) 0.717 肿瘤位置 胸中上段 参照 — 参照 — 胸下段 1.268(0.635~2.531) 0.501 1.071(0.586~1.957) 0.825 分化程度 高分化 参照 — 参照 — 中分化 0.329(0.075~1.443) 0.141 0.299(0.088~1.025) 0.055 低分化 0.552(0.122~2.505) 0.441 0.455(0.129~1.614) 0.223 脉管侵犯 无 参照 — 参照 — 有 1.044(0.496~2.201) 0.909 1.278(0.674~2.420) 0.452 神经侵犯 无 参照 — 参照 — 有 0.848(0.254~2.824) 0.788 0.959(0.375~2.457) 0.931 肿瘤直径 < 3.25 cm 参照 — 参照 — ≥3.25 cm 4.362(1.958~9.717) < 0.001 2.265(1.217~4.214) 0.010 T分期 T1~T2期 参照 — 参照 — T3期 5.036(2.063~12.293) < 0.001 5.711(2.653~12.294) < 0.001 N分期 N0期 参照 — 参照 — N1~N2期 0.817(0.406~1.643) 0.57 0.841(0.459~1.542) 0.576 pTNM分期 Ⅱ期 参照 — 参照 — Ⅲ期 0.861(0.428~1.732) 0.674 0.893(0.487~1.638) 0.715 手术方式 胸腔镜 参照 — 参照 — 开胸手术 1.589(0.655~3.855) 0.306 1.086(0.458~2.576) 0.851 同期化疗 无 参照 — 参照 — 有 1.328(0.664~2.657) 0.423 1.557(0.850~2.852) 0.151 化疗方案 未同期化疗 参照 — 参照 — 紫杉醇联合顺铂方案 2.109(0.827~5.377) 0.118 1.601(0.680~3.772) 0.282 氟尿嘧啶联合顺铂方案 0.983(0.226~4.271) 0.982 0.730(0.171~3.106) 0.670 替吉奥单药方案 1.302(0.538~3.151) 0.559 1.914(0.934~3.921) 0.076 PIV 高 参照 — 参照 — 低 0.244(0.119~0.500) < 0.001 0.340(0.184~0.627) 0.001 HR: 风险比; CI: 置信区间; pTNM分期: 手术后TNM分期; PIV: 泛免疫炎症值。 表 4 食管癌术后辅助放疗患者总生存率和无病生存率的多因素分析
因素 分类 总生存率HR(95%CI) P 无病生存率HR(95%CI) P 肿瘤直径 < 3.25 cm 参照 — 参照 — ≥3.25 cm 2.554(1.096~5.953) 0.030 1.188(0.614~2.298) 0.609 T分期 T1~T2期 参照 — 参照 — T3期 3.389(1.344~8.542) 0.010 5.059(2.225~11.505) < 0.001 PIV 高 参照 — 参照 — 低 0.321(0.154~0.669) 0.002 0.398(0.212~0.750) 0.004 HR: 风险比; CI: 置信区间; PIV: 泛免疫炎症值。 -
[1] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] UHLENHOPP D J, THEN E O, SUNKARA T, et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors[J]. Clin J Gastroenterol, 2020, 13(6): 1010-1021. doi: 10.1007/s12328-020-01237-x
[3] WATANABE M, OTAKE R, KOZUKI R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer[J]. Surg Today, 2020, 50(1): 12-20. doi: 10.1007/s00595-019-01878-7
[4] EYCK B M, VAN LANSCHOT J J B, HULSHOF M C C M, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial[J]. J Clin Oncol, 2021, 39(18): 1995-2004. doi: 10.1200/JCO.20.03614
[5] KAKEJI Y, OSHIKIRI T, TAKIGUCHI G, et al. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy[J]. Esophagus, 2021, 18(1): 25-32. doi: 10.1007/s10388-020-00782-1
[6] GRIVENNIKOV S I, GRETEN F R, KARIN M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140(6): 883-899. doi: 10.1016/j.cell.2010.01.025
[7] WINARNO G N A, MULYANTARI A I, KURNIADI A, et al. Predicting chemotherapy resistance in gestational trophoblastic neoplasia: ratio of neutrophils, lymphocytes, monocytes, and platelets[J]. Med Sci Monit, 2022, 28: e938499.
[8] PORTALE G, BARTOLOTTA P, AZZOLINA D, et al. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: systematic review and meta-analysis[J]. Langenbecks Arch Surg, 2023, 408(1): 85. doi: 10.1007/s00423-023-02786-8
[9] ZHANG H D, SHANG X B, REN P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma[J]. J Cell Physiol, 2019, 234(2): 1794-1802. doi: 10.1002/jcp.27052
[10] GAO Y B, GUO W, CAI S H, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma[J]. J Cancer, 2019, 10(14): 3188-3196. doi: 10.7150/jca.30281
[11] CAO Z, JI J, ZHANG C, et al. The preoperative neutrophil-to-lymphocyte ratio is not a marker of prostate cancer characteristics but is an independent predictor of biochemical recurrence in patients receiving radical prostatectomy[J]. Cancer Med, 2019, 8(3): 1004-1012. doi: 10.1002/cam4.1984
[12] FUCÀ G, GUARINI V, ANTONIOTTI C, et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials[J]. Br J Cancer, 2020, 123(3): 403-409. doi: 10.1038/s41416-020-0894-7
[13] SUSOK L, SAID S, REINERT D, et al. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy[J]. J Cancer Res Clin Oncol, 2022, 148(11): 3103-3108. doi: 10.1007/s00432-021-03878-y
[14] CHEN X R, HONG X C, CHEN G, et al. The Pan-Immune-Inflammation Value predicts the survival of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer treated with first-line ALK inhibitor[J]. Transl Oncol, 2022, 17: 101338. doi: 10.1016/j.tranon.2021.101338
[15] YEKEDÜZ E, TURAL D, ERTÜRK Ī, et al. The relationship between pan-immune-inflammation value and survival outcomes in patients with metastatic renal cell carcinoma treated with nivolumab in the second line and beyond: a Turkish oncology group kidney cancer consortium (TKCC) study[J]. J Cancer Res Clin Oncol, 2022, 148(12): 3537-3546. doi: 10.1007/s00432-022-04055-5
[16] YAZGAN S C, YEKEDVZ E, UTKAN G, et al. Prognostic role of pan-immune-inflammation value in patients with metastatic castration-resistant prostate cancer treated with androgen receptor-signaling inhibitors[J]. Prostate, 2022, 82(15): 1456-1461. doi: 10.1002/pros.24419
[17] LIGORIO F, FUCÀG, ZATTARIN E, et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2(HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab[J]. Cancers, 2021, 13(8): 1964. doi: 10.3390/cancers13081964
[18] RICE T W, ISHWARAN H, HOFSTETTER W L, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals[J]. Dis Esophagus, 2016, 29(8): 897-905. doi: 10.1111/dote.12533
[19] ELINAV E, NOWARSKI R, THAISS C A, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms[J]. Nat Rev Cancer, 2013, 13(11): 759-771. doi: 10.1038/nrc3611
[20] LIN F, ZHANG L P, XIE S Y, et al. Pan-immune-inflammation value: a new prognostic index in operative breast cancer[J]. Front Oncol, 2022, 12: 830138. doi: 10.3389/fonc.2022.830138
[21] SATO S, SHIMIZU T, ISHIZUKA M, et al. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage Ⅰ-Ⅲ colorectal cancer patients undergoing surgery[J]. Surg Today, 2022, 52(8): 1160-1169. doi: 10.1007/s00595-021-02448-6
[22] PROVENZANO L, LOBEFARO R, LIGORIO F, et al. The pan-immune-inflammation value is associated with clinical outcomes in patients with advanced TNBC treated with first-line, platinum-based chemotherapy: an institutional retrospective analysis[J]. Ther Adv Med Oncol, 2023, 15: 17588359231165978.
[23] YANG X C, LIU H, LIU D C, et al. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: a systematic review and meta-analysis[J]. Front Oncol, 2022, 12: 1036890. doi: 10.3389/fonc.2022.1036890
[24] GUVEN D C, SAHIN T K, ERUL E, et al. The association between the pan-immune-inflammation value and cancer prognosis: a systematic review and meta-analysis[J]. Cancers, 2022, 14(11): 2675. doi: 10.3390/cancers14112675
[25] FENG J F, WANG L, YANG X, et al. Clinical utility of preoperative pan-immune-inflammation value (PIV) for prognostication in patients with esophageal squamous cell carcinoma[J]. Int Immunopharmacol, 2023, 123: 110805. doi: 10.1016/j.intimp.2023.110805
[26] WU X, LI S Q, CHEN D J, et al. An inflammatory response-related gene signature associated with immune status and prognosis of acute myeloid leukemia[J]. Am J Transl Res, 2022, 14(7): 4898-4917.
[27] XU X R, YOUSEF G M, NI H Y. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents[J]. Blood, 2018, 131(16): 1777-1789. doi: 10.1182/blood-2017-05-743187
[28] SHAUL M E, FRIDLENDER Z G. Neutrophils as active regulators of the immune system in the tumor microenvironment[J]. J Leukoc Biol, 2017, 102(2): 343-349. doi: 10.1189/jlb.5MR1216-508R
[29] YANG L, ZHANG Y. Tumor-associated macrophages: from basic research to clinical application[J]. J Hematol Oncol, 2017, 10(1): 58. doi: 10.1186/s13045-017-0430-2
[30] LIN B S, DU L K, LI H M, et al. Tumor-infiltrating lymphocytes: Warriors fight against tumors powerfully[J]. Biomed Pharmacother, 2020, 132: 110873. doi: 10.1016/j.biopha.2020.110873




 下载:
下载:

 苏公网安备 32100302010246号
苏公网安备 32100302010246号
