Effects of icaritin on proliferation, apoptosis, migration and invasion of human tongue squamous cell CAL-27
-
摘要:目的
探索淫羊藿素对人舌鳞癌细胞CAL-27的增殖、凋亡、迁移和侵袭的影响及可能作用机制。
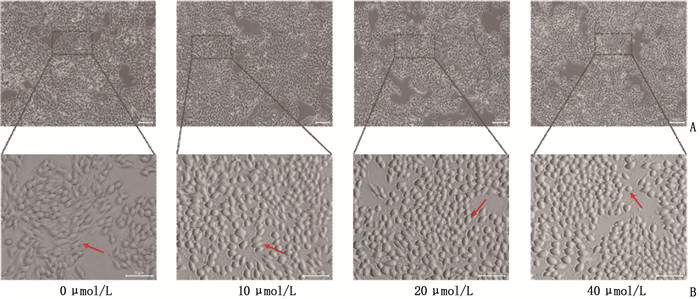
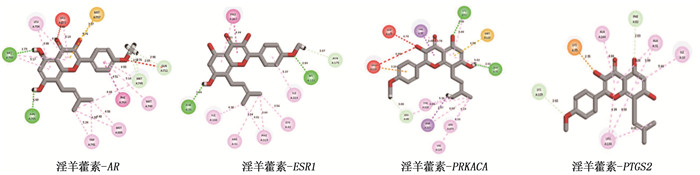
方法分别采用0、10、20、40 μmol/L浓度的淫羊藿素干预人舌鳞癌细胞CAL-27。采用CCK-8法及克隆形成实验检测细胞增殖; 采用划痕实验检测细胞迁移; 采用流式细胞术检测细胞凋亡; 采用Transwell实验检测细胞迁移和侵袭。采用分子对接预测淫羊藿素与舌鳞癌目的基因结合效果, 采用逆转录-实时定量聚合酶链反应(RT-qPCR)进行验证。
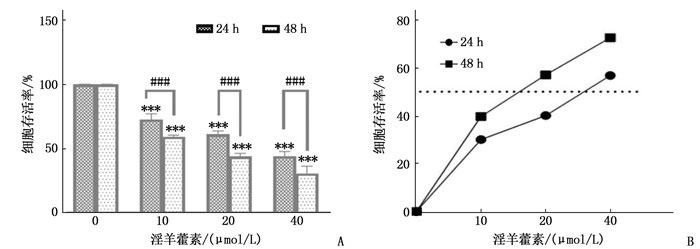
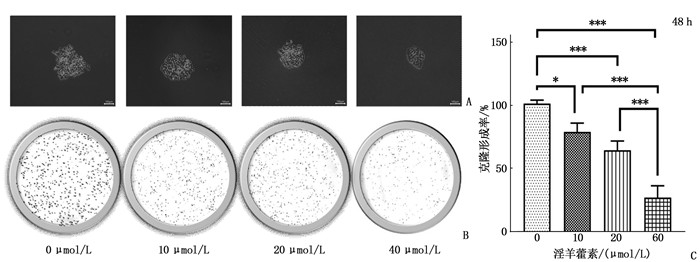
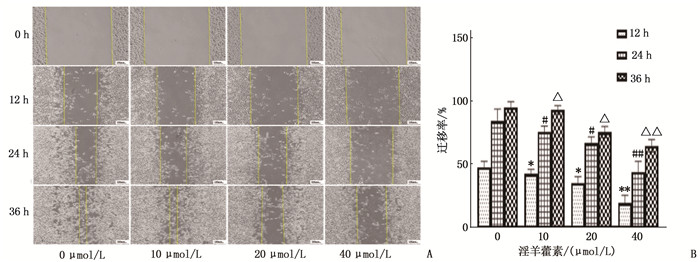
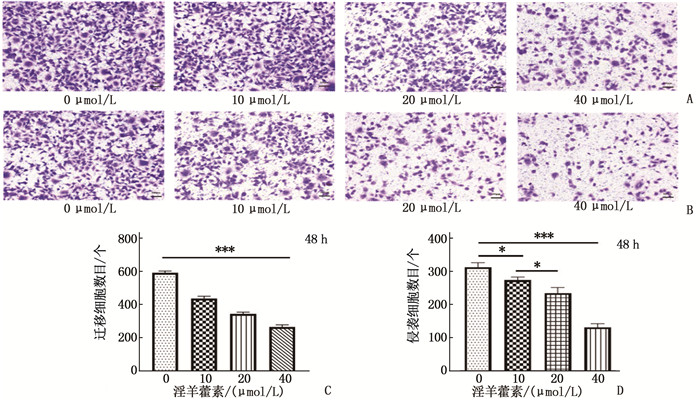
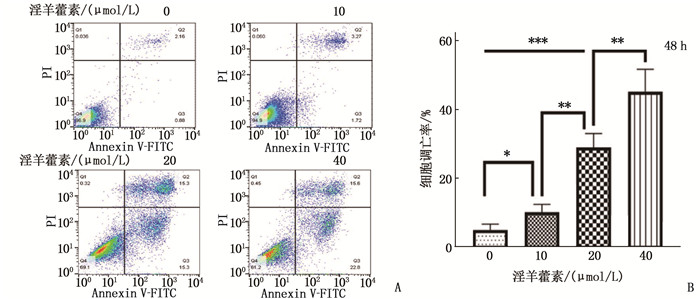
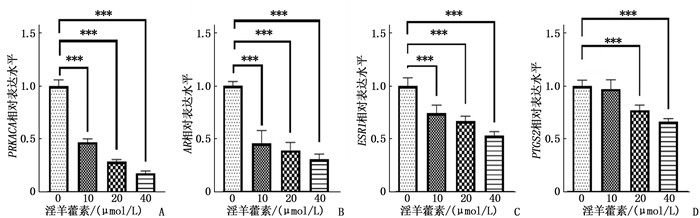
结果淫羊藿素对CAL-27细胞作用24 h的半数抑制浓度(IC50)为33.37 μmol/L, 48 h IC50为15.57 μmol/L。与0 μmol/L浓度淫羊藿素相比, 40 μmol/L浓度的CAL-27细胞克隆形成率降低,细胞凋亡率升高,差异有统计学意义(P < 0.001)。淫羊藿素浓度增加时, CAL-27的迁移、侵袭数呈淫羊藿素剂量依赖性抑制; 细胞内AR、ESR1、PRKACA、PTGS2的相对表达量呈淫羊藿素剂量依赖性减少。
结论淫羊藿素可抑制人舌鳞癌细胞CAL-27的增殖、迁移、侵袭, 促进细胞凋亡,其机制可能与淫羊藿素影响细胞CAL-27的AR、ESR1、PRKACA的表达有关。
Abstract:ObjectiveTo explore the effect of icaritin on proliferation, apoptosis, migration and invasion of human tongue squamous cell CAL-27 and its possible mechanism.
MethodsHuman tongue squamous cell CAL-27 was treated with icaritin at concentrations of 0, 10, 20 and 40 μmol/L, respectively. Cell proliferation was detected by CCK-8 assay and clonal formation assay; cell migration was detected by scratch test; the apoptosis was detected by flow cytometry; the Transwell assay was used to detect cell migration and invasion. Molecular docking was used to predict the binding effect of icaritin to the target gene of tongue squamous cell carcinoma, which was verified by quantitative reverse transcription-polymerase chain reaction (RT-qPCR).
ResultsThe median inhibitory concentration (IC50) of icaritin on CAL-27 cells was 33.37 μmol/L for 24 h and 15.57 μmol/L for 48 h. Compared with 0 μmol/L concentration of icaritin, the clonal formation rate of CAL-27 cells at 40 μmol/L concentration was significantly decreased, and the apoptosis rate was significantly increased (P < 0.001). When icaritin concentration increased, the number of CAL-27 for migration and invasion was dose-dependently inhibited; the relative expression levels of AR, ESR1, PRKACA and PTGS2 decreased in a dose-dependent manner.
ConclusionIcaritin can inhibit the proliferation, migration, invasion and apoptosis of human tongue squamous cell CAL-27, and the mechanism may be related to the effect of icaritin on the expression of AR, ESR1 and PRKACA in cell CAL-27.
-
Keywords:
- icaritin /
- tongue squamous cell carcinoma /
- proliferation /
- apoptosis /
- migration /
- invasion /
- mechanism
-
-
表 1 RT-qPCR检测所用引物序列
基因 引物序列(5′-3′) AR F: CAGCAGCAGCAGCAAGAGACTAG R: CCTCATCCAGGACCAGGTAGCC ESR1 F: CCTCCTCATCCTCTCCCACATCAG R: GCATCTCCAGCAGCAGGTCATAG PRKACA F: TCGCAGACCAGCCCATCCAG R: GTTCCGCAGCAGGTCCTTCAAG PTGS2 F: GGGTTGCTGGTGGTAGGAATGTTC R: CTGGTATTTCATCTGCCTGCTCTGG GAPDH F: TCGTGGAAGGACTCATGACC R: TCCACCACCCTGTTGCTGTA -
[1] 吴开柳, 李思毅, 张陈平. 舌鳞状细胞癌颈淋巴结转移的特点和评估处理[J]. 国际口腔医学杂志, 2015, 42(1): 119-122. https://www.cnki.com.cn/Article/CJFDTOTAL-GWKQ201501037.htm [2] 夏晓芳, 张文颖, 袁海花, 等. 术前预后营养指数对首次行根治性手术的口腔鳞状细胞癌患者预后的预测价值[J]. 实用临床医药杂志, 2021, 25(8): 6-10. doi: 10.7619/jcmp.20210831 [3] SHI J X, DONG Y, JIANG W Y, et al. MRI-based peritumoral radiomics analysis for preoperative prediction of lymph node metastasis in early-stage cervical cancer: a multi-center study[J]. Magn Reson Imaging, 2022, 88: 1-8. doi: 10.1016/j.mri.2021.12.008
[4] 许刚, 唐春梅, 赵会杰. 口腔颌面部鳞状细胞癌患者不同原发灶部位与颈部Ⅰ、Ⅱ、Ⅲ、Ⅳ、Ⅴ区转移的相关性[J]. 实用临床医药杂志, 2017, 21(9): 116-118. doi: 10.7619/jcmp.201709030 [5] ZHOU M, ZHENG W, SUN X G, et al. Comparative analysis of chemical components in different parts of Epimedium Herb[J]. J Pharm Biomed Anal, 2021, 198: 113984. doi: 10.1016/j.jpba.2021.113984
[6] ARIEF Z M, MUNSHI A H, SHAWL A S. Evaluation of medicinal value of Epimedium elatum on the basis of pharmacologically active constituents, Icariin and Icariside-Ⅱ[J]. Pakistan journal of pharmaceutical ences, 2015, 28(5): 1665-1669.
[7] LEI K, MA B, SHI P, et al. Icariin mitigates the growth and invasion ability of human oral squamous cell carcinoma via inhibiting toll-like receptor 4 and phosphorylation of NF-κB P65[J]. Onco Targets Ther, 2020, 13: 299-307. doi: 10.2147/OTT.S214514
[8] YANG J G, ZHANG J, CHEN X J, et al. Stable loading and delivery of icaritin using PEG-PCL micelles for effective treatment of oral squamous cell carcinoma[J]. Curr Drug Deliv, 2021, 18(7): 975-983. doi: 10.2174/1567201818999201210211636
[9] 杨建光. 淫羊藿素抗口腔鳞状细胞癌的作用及机制研究[D]. 武汉: 武汉大学, 2017. [10] 王大伟, 邓秀兰, 牛建昭, 等. 淫羊藿素和脱水淫羊藿素对人类乳腺癌细胞T47D增殖和细胞周期的影响[J]. 北京中医药, 2009, 28(8): 637-640. doi: 10.16025/j.1674-1307.2009.08.032 [11] NGUYEN V S, SHI L, WANG S C, et al. Synthesis of icaritin and β-anhydroicaritin mannich base derivatives and their cytotoxic activities on three human cancer cell lines[J]. Anticancer Agents Med Chem, 2017, 17(1): 137-142. doi: 10.2174/1871520616666160404111210
[12] YU Z, GUO J F, HU M Y, et al. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma[J]. ACS Nano, 2020, 14(4): 4816-4828. doi: 10.1021/acsnano.0c00708
[13] XIE Y Z, XIE L, CHEN A L, et al. Anti-HIV/SIV activity of icariin and its metabolite anhydroicaritin mainly involve reverse transcriptase[J]. Eur J Pharmacol, 2020, 884: 173327. doi: 10.1016/j.ejphar.2020.173327
[14] FAISAL M, DHANANI R, ULLAH S, et al. Prognostic outcomes of treatment naïve oral tongue squamous cell carcinoma (OTSCC): a comprehensive analysis of 14 years[J]. Eur Arch Oto Rhino Laryngol, 2021, 278(8): 3045-3053. doi: 10.1007/s00405-020-06482-x
[15] 潘朝斌. 舌鳞癌的临床综合序列治疗研究进展[J]. 口腔疾病防治, 2018, 26(5): 273-280. https://www.cnki.com.cn/Article/CJFDTOTAL-GDYB201805001.htm [16] MEHTA K A, PATEL K A, KUNNUMAKKARA A B, et al. Curbing the deregulation of glycosylation in tongue carcinoma cells with natural compounds[J]. Anticancer Agents Med Chem, 2021, 21(13): 1717-1723. doi: 10.2174/1871520620999201124213259
[17] 陈思宇, 付帅, 吴勇. 醋酸棉酚对人舌鳞癌Cal-27细胞侵袭性作用的实验研究[J]. 昆明医科大学学报, 2020, 41(11): 12-17. https://www.cnki.com.cn/Article/CJFDTOTAL-KMYX202011003.htm [18] 周鑫. 阿可拉定联合常规方案治疗晚期恶性实体肿瘤疗效的相关性研究[D]. 北京: 中国人民解放军医学院, 2019. [19] 周鑫, 赵卫红, 赵晓, 等. 阿可拉定联合常规方案治疗晚期非小细胞肺癌的疗效和安全性评价[J]. 解放军医学院学报, 2019, 40(3): 219-222. https://www.cnki.com.cn/Article/CJFDTOTAL-JYJX201903006.htm [20] ZHAO X Q, LIN Y, JIANG B J, et al. Icaritin inhibits lung cancer-induced osteoclastogenesis by suppressing the expression of IL-6 and TNF-a and through AMPK/mTOR signaling pathway[J]. Anticancer Drugs, 2020, 31(10): 1004-1011. doi: 10.1097/CAD.0000000000000976
[21] 王雪. 阿可拉定对三阴性乳腺癌的抑制作用及机制研究[D]. 武汉: 武汉大学, 2017. [22] ZHANG Y, PAN T C, ZHONG X X, et al. Androgen receptor promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 2[J]. Tumour Biol, 2015, 36(8): 5859-5864. doi: 10.1007/s13277-015-3257-x
[23] WU T F, LUO F J, CHANG Y L, et al. The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells[J]. Oral Dis, 2015, 21(3): 320-327. doi: 10.1111/odi.12272
[24] RADES D, SEIBOLD N D, SCHILD S E, et al. Androgen receptor expression: prognostic value in locally advanced squamous cell carcinoma of the head and neck[J]. Al, 2013, 189(10): 849-855.
[25] ADNAN Y, ALI S M A, AWAN M S, et al. Hormone receptors AR, ER, PR and growth factor receptor Her-2 expression in oral squamous cell carcinoma: correlation with overall survival, disease-free survival and 10-year survival in a high-risk population[J]. PLoS One, 2022, 17(5): e0267300. doi: 10.1371/journal.pone.0267300
[26] CARAUSU M, BIDARD F C, CALLENS C, et al. ESR1 mutations: a new biomarker in breast cancer[J]. Expert Rev Mol Diagn, 2019, 19(7): 599-611.
[27] KWON S, AHN S H, JEONG W J, et al. Estrogen receptor α as a predictive biomarker for survival in human papillomavirus-positive oropharyngeal squamous cell carcinoma[J]. J Transl Med, 2020, 18(1): 240.
[28] VERMA A, SCHWARTZ N, COHEN D J, et al. Estrogen signaling and estrogen receptors as prognostic indicators in laryngeal cancer[J]. Steroids, 2019, 152: 108498.
[29] TURNHAM R E, SCOTT J D. Protein kinase A catalytic subunit isoform PRKACA; History, function and physiology[J]. Gene, 2016, 577(2): 101-108.
[30] KASTENHUBER E R, LALAZAR G, HOULIHAN S L, et al. DNAJB1-PRKACA fusion kinase interacts with β-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma[J]. Proc Natl Acad Sci U S A, 2017, 114(50): 13076-13084.
[31] YANG F, XIE H Y, YANG L F, et al. Stabilization of MORC2 by estrogen and antiestrogens through GPER1-PRKACA-CMA pathway contributes to estrogen-induced proliferation and endocrine resistance of breast cancer cells[J]. Autophagy, 2020, 16(6): 1061-1076.
[32] ZAPPAVIGNA S, COSSU A M, GRIMALDI A, et al. Anti-inflammatory drugs as anticancer agents[J]. Int J Mol Sci, 2020, 21(7): 2605.
[33] KUNZMANN A T, MURRAY L J, CARDWELL C R, et al. PTGS2 (Cyclooxygenase-2) expression and survival among colorectal cancer patients: a systematic review[J]. Cancer Epidemiol Biomarkers Prev, 2013, 22(9): 1490-1497.
[34] ZHANG Z L. miR-124-3p suppresses prostatic carcinoma by targeting PTGS2 through the AKT/NF-κB pathway[J]. Mol Biotechnol, 2021, 63(7): 621-630.
[35] YE Y, WANG X P, JESCHKE U, et al. COX-2-PGE2-EPs in gynecological cancers[J]. Arch Gynecol Obstet, 2020, 301(6): 1365-1375.
[36] FENG B H, SHEN Y, PASTOR HOSTENCH X, et al. Integrative analysis of multi-omics data identified EGFR and PTGS2 as key nodes in a gene regulatory network related to immune phenotypes in head and neck cancer[J]. Clin Cancer Res, 2020, 26(14): 3616-3628.
-
期刊类型引用(7)
1. 冯晓霞,陈玉仙,张晓萍. 妊娠期肝内胆汁淤积症合并乙型肝炎病毒感染与患者妊娠结局相关性研究. 陕西医学杂志. 2025(02): 227-230 .  百度学术
百度学术
2. 刘佳,于雅彬,王兰,李英,于欣,蔡永艳,马娜,阎志新. 重症腺病毒肺炎患儿凝血功能和免疫功能特征及其临床意义. 实用临床医药杂志. 2025(05): 122-126 .  本站查看
本站查看
3. 王晶晶,李萍,高尚啸,周玥. 阶梯护理决策辅助干预在妊娠期肝内胆汁淤积症患者中的应用. 国际护理学杂志. 2024(09): 1625-1629 .  百度学术
百度学术
4. 宋对对,牛丽娜,张小娟,马萍,卫晓娟. 妊娠期肝内胆汁淤积症患者不良妊娠结局影响因素. 中国计划生育学杂志. 2024(07): 1586-1590 .  百度学术
百度学术
5. 潘伟杰,程福安,李婉莹,林炳柱. NLR和凝血四项与妊娠期肝内胆汁淤积症病情程度的相关性及检测价值. 实用检验医师杂志. 2024(03): 222-224 .  百度学术
百度学术
6. 陈慧. 妊娠期肝内胆汁淤积症孕妇血清总胆酸水平和胎儿心脏功能的相关性. 临床研究. 2023(09): 44-46 .  百度学术
百度学术
7. 罗钊琰,唐征宇. 茵陈蒿汤加味辅助治疗湿热郁结型妊娠肝内胆汁淤积症的临床效果. 中国社区医师. 2023(36): 64-66 .  百度学术
百度学术
其他类型引用(1)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号