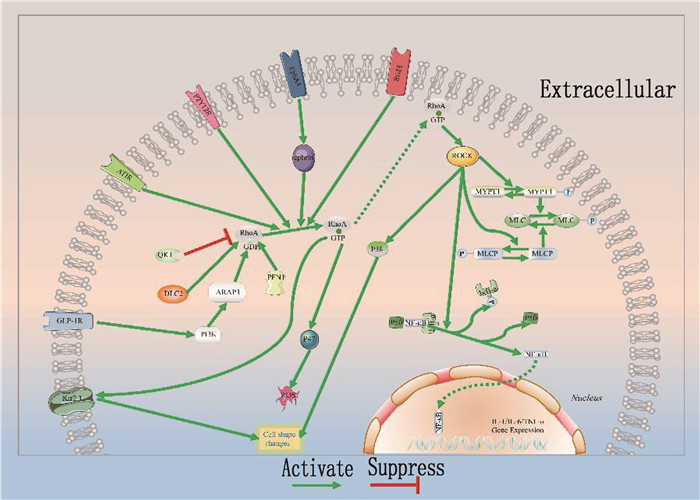
Research progress in regulation of microglial polarization by RhoA/ROCK signaling pathway
-
摘要:
小胶质细胞广泛参与中枢神经系统的多种病理生理过程, 其极化特性则与神经元的炎症反应和损伤修复密切相关。Ras同源基因家族蛋白A(RhoA)/Rho相关卷曲螺旋蛋白激酶(ROCK)信号通路对小胶质细胞极化具有重要的调控功能。目前,通过促进小胶质细胞M2型极化治疗神经元受损的相关研究已成为神经科学领域的热点之一,但RhoA/ROCK通路对极化的调控作用仍未明确。本文综述RhoA/ROCK信号通路对小胶质细胞极化的影响,以期为脑保护分子机制研究及临床治疗提供新思路。
Abstract:Microglia are widely involved in various pathophysiological processes of the central nervous system, and their polarization characteristics are closely related to the inflammatory response and injury repair of neurons. Ras homologous gene family protein A (RhoA)/Rho-associated coiled-coil protein kinase (ROCK) signaling pathway plays an important role in regulating microglia polarization. At present, the related research on the treatment of neuronal damage by promoting microglial M2-type polarization has become one of the hot spots in the field of neuroscience, but the effect of RhoA/ROCK pathway on polarization is still unclear. This article reviewed the effect of RhoA/ROCK signaling pathway on microglial polarization in order to provide new ideas for the study of molecular mechanism of brain protection and clinical treatment.
-
Keywords:
- Rho factor /
- Rho-associated kinases /
- microglia /
- polarization /
- inflammation /
- cerebral injury
-
反复呼吸道感染(RRI)是指上下呼吸道感染次数超过一定范围,在儿童中的发病率较高[1]。RRI的发生与儿童免疫力低下、长期挑食、居住环境不良及微量元素缺乏等因素密切相关,临床表现包括鼻塞、发热、咳嗽、食欲不振和腹泻等[2-3]。RRI通常起病较急,若治疗不及时或不当,会严重影响儿童的生长发育。因此,探讨与RRI患儿病情严重程度及预后相关的指标,对于优化临床治疗效果及改善预后具有重要意义[4]。既往研究[5-7]报道,内皮细胞特异性分子-1(ESM-1, 又称endocan)、白细胞介素(IL)-1α和人类软骨糖蛋白-39(又称YKL-40)在呼吸道疾病的发生和进展中扮演重要角色。然而, endocan、IL-1α和YKL-40与RRI患儿预后的关系尚不明确。因此,本研究分析血清endocan、IL-1α及YKL-40水平与RRI患儿病情严重程度和预后的关系,以期为改善RRI患儿的预后提供参考依据,现报告如下。
1. 资料与方法
1.1 一般资料
前瞻性选取2022年10月—2023年9月青岛市第八人民医院收治的86例RRI患儿作为病例组,并根据病情严重程度[8]分为轻度组37例、中度组31例和重度组18例。纳入标准: ①符合RRI诊断标准[9]者; ②年龄1~12岁者; ③病程1年及以上者。排除标准: ①不接受随访者; ②合并其他慢性感染疾病者; ③既往有肿瘤史者; ④有免疫功能缺陷者; ⑤伴有血液类疾病者; ⑥有肺部手术史者; ⑦合并重要脏器功能障碍者; ⑧近3个月内使用激素或影响免疫功能的药物者; ⑨先天性呼吸道畸形者。另选取同期体检的94例健康儿童作为对照组。本研究经青岛市第八人民医院医学伦理委员会审核通过(批号: 20211220-02), 患儿家属均签署知情同意书。
1.2 方法
1.2.1 血清endocan、IL-1α和YKL-40水平检测
所有儿童于入院后抽取空腹静脉血3 mL, 血液经离心处理后,采用酶联免疫吸附试验(ELISA)检测血清中endocan、IL-1α和YKL-40水平。操作严格按照人endocan(货号KOA0444, 艾美捷科技有限公司)、IL-1α(货号KS016981, 上海科顺生物科技有限公司)、YKL-40(货号YS02583B, 上海雅吉生物科技有限公司)ELISA试剂盒说明书进行,使用Multiskan SkyHigh酶标仪(赛默飞)检测各样品在450 nm处的吸光度,并根据标准曲线计算浓度。
1.2.2 随访及预后评估
所有患儿接受常规抗感染和对症治疗,若治疗结束后6个月内呼吸道感染次数减少≥50%则归为预后良好组(n=64), 否则归为预后不良组(n=22)。
1.3 统计学分析
采用SPSS 25.0统计学软件进行数据处理。计数资料以[n(%)]表示,比较采用χ2检验。计量资料以(x±s)表示,比较采用t检验或单因素方差分析。采用Spearman相关性分析法评估血清endocan、IL-1α和YKL-40水平与RRI患儿病情严重程度的相关性; 采用多因素Logistic回归分析探讨RRI患儿预后的影响因素; 绘制受试者工作特征(ROC)曲线,分析血清endocan、IL-1α和YKL-40对RRI患儿预后的预测价值。P < 0.05表示差异有统计学意义。
2. 结果
2.1 病例组和对照组一般资料及血清endocan、IL-1α、YKL-40水平比较
病例组在年龄、体质量指数、性别方面与对照组比较,差异无统计学意义(P>0.05); 病例组血清endocan、IL-1α、YKL-40水平均高于对照组,差异有统计学意义(P < 0.05), 见表 1。
表 1 病例组与对照组一般资料及血清endocan、IL-1α、YKL-40水平比较(x±s)[n(%)]指标 病例组(n=86) 对照组(n=94) t/χ2 P 年龄/岁 7.16±1.61 7.63±1.85 1.811 0.072 体质量指数/(kg/m2) 21.80±2.30 21.49±2.18 0.928 0.355 endocan/(ng/mL) 11.43±2.70 8.05±2.16 9.311 < 0.001 IL-1α/(pg/mL) 8.29±2.15 4.61±0.94 15.096 < 0.001 YKL-40/(ng/mL) 37.14±5.37 25.47±4.12 16.437 < 0.001 性别 女 50(58.14) 52(55.32) 0.145 0.703 男 36(41.86) 42(44.68) endocan: 又称内皮细胞特异性分子-1; IL-1α: 白细胞介素-1α;
YKL-40: 又称人类软骨糖蛋白-39。2.2 轻度组、中度组和重度组血清endocan、IL-1α、YKL-40水平比较
中度组和重度组患儿血清endocan、IL-1α、YKL-40水平均高于轻度组,且重度组患儿水平高于中度组,差异有统计学意义(P < 0.05), 见表 2。
表 2 轻度组、中度组和重度组患儿血清endocan、IL-1α、YKL-40水平比较(x±s)组别 n endocan/(ng/mL) IL-1α/(pg/mL) YKL-40/(ng/mL) 轻度组 37 8.61±2.52 6.03±1.96 31.18±5.04 中度组 31 12.05±2.70* 8.64±2.27* 39.12±5.47* 重度组 18 16.14±3.08*# 12.35±2.32*# 45.98±5.86*# 与轻度组比较, * P < 0.05; 与中度组比较, #P < 0.05。 2.3 血清endocan、IL-1α、YKL-40水平与RRI患儿病情严重程度的相关性
Spearman相关性分析结果显示,血清endocan、IL-1α、YKL-40水平与RRI患儿病情严重程度均呈显著正相关(r=0.407、0.493、0.452, P < 0.001)。
2.4 预后良好组和预后不良组血清endocan、IL-1α、YKL-40水平比较
预后不良组患儿血清endocan、IL-1α、YKL-40水平均高于预后良好组,差异有统计学意义(P < 0.05), 见表 3。
表 3 预后良好组和预后不良组血清endocan、IL-1α、YKL-40水平比较(x±s)组别 n endocan/(ng/mL) IL-1α/(pg/mL) YKL-40/(ng/mL) 预后良好组 64 10.24±2.43 7.18±1.87 34.91±5.08 预后不良组 22 14.89±3.47* 11.52±2.95* 43.64±6.23* 与预后良好组比较, * P < 0.05。 2.5 预后良好组和预后不良组临床资料比较
预后良好组和预后不良组患儿在年龄、体质量指数、病程、居住地、早产、被动烟草暴露等方面比较,差异均无统计学意义(P>0.05), 见表 4。
表 4 预后良好组和预后不良组患儿临床资料比较(x±s)[n(%)]指标 分类 预后良好组
(n=64)预后不良组
(n=22)t/χ2 P 年龄/岁 7.29±1.64 6.83±1.52 1.155 0.251 体质量指数(kg/m2) 21.95±2.28 21.37±2.34 1.023 0.309 病程/月 9.13±2.37 10.14±2.46 1.708 0.091 居住地 城镇/城市 39(60.94) 12(54.55) 0.277 0.599 农村 25(39.06) 10(45.45) 性别 女 36(56.25) 14(63.64) 0.367 0.545 男 28(43.75) 8(36.36) 早产 是 14(21.88) 7(31.82) 0.877 0.349 否 50(78.12) 15(68.18) 病情严重程度 轻度 31(48.44) 6(27.28) 3.726 0.155 中度 23(35.94) 8(36.36) 重度 10(15.62) 8(36.36) 被动烟草暴露 是 16(25.00) 9(40.91) 2.010 0.156 否 48(75.00) 13(59.09) 2.6 RRI患儿预后的多因素Logistic回归分析
将RRI患儿预后作为因变量(不良=1, 良好=0), 将血清endocan、IL-1α、YKL-40作为自变量(均为连续变量),纳入多因素Logistic回归模型。分析结果显示,血清endocan(OR=1.412)、IL-1α(OR=1.583)、YKL-40(OR=1.259)均为RRI患儿预后的独立影响因素(P < 0.05), 见表 5。
表 5 RRI患儿预后的多因素Logistic回归分析变量 β SE Wald χ2 P OR 95%CI endocan 0.345 0.114 9.159 0.002 1.412 1.129~1.766 IL-1α 0.459 0.128 12.877 < 0.001 1.583 1.232~2.034 YKL-40 0.230 0.086 7.172 0.007 1.259 1.064~1.490 2.7 血清endocan、IL-1α、YKL-40对RRI患儿预后的预测价值
以RRI患儿预后为因变量,以血清endocan、IL-1α、YKL-40为检验变量,绘制ROC曲线。分析结果显示,血清endocan、IL-1α、YKL-40预测RRI患儿预后不良的曲线下面积(AUC)分别为0.777、0.795、0.787, 敏感度分别为72.73%、68.18%、72.73%, 特异度分别为84.37%、82.81%、82.81%; 血清endocan、IL-1α、YKL-40联合预测RRI患儿预后不良的敏感度为90.91%, 特异度为81.25%, AUC为0.925, 显著大于endocan、IL-1α、YKL-40单独预测的AUC(Z=2.217、2.169、2.218, P=0.027、0.030、0.027), 见图 1、表 6。
表 6 血清endocan、IL-1α、YKL-40对RRI患儿预后的预测效能变量 曲线下面积 截断值 95%CI 敏感度/% 特异度/% 约登指数 endocan 0.777 12.85 ng/mL 0.674~0.860 72.73 84.37 0.571 IL-1α 0.795 9.63 pg/mL 0.694~0.874 68.18 82.81 0.510 YKL-40 0.787 37.59 ng/mL 0.686~0.868 72.73 82.81 0.555 三者联合 0.925 — 0.848~0.971 90.91 81.25 0.722 3. 讨论
儿童气管、气道尚处于发育阶段,易受病毒、细菌等病原体入侵,引发呼吸道感染[10]。RRI往往伴随炎症反应的发生,其病情与炎症反应程度密切相关[11-13]。endocan、IL-1α、YKL-40与机体炎症通路紧密相关,因此,探讨血清中endocan、IL-1α、YKL-40水平与RRI患儿病情及预后的关系具有重要意义。
endocan是由内皮细胞分泌的蛋白多糖,主要在肾和肺内皮细胞表达,人类endocan编码基因位于5号染色体上[14]。endocan能够影响白细胞的黏附与迁移,在炎性因子作用下其表达量升高[15]。叶云虹等[16]报道,脓毒症患者endocan表达上调,推测脓毒症炎性级联反应导致肺血管内皮细胞受损,进而诱导endocan表达。本研究结果显示, RRI患儿血清endocan水平显著高于健康儿童,表明RRI会导致endocan水平升高。进一步分析发现, RRI患儿血清endocan水平随RRI病情严重程度的增加而升高,且相关性分析显示RRI患儿血清endocan与RRI病情严重程度呈显著正相关,提示RRI患儿病情越严重,内皮细胞功能障碍程度越高。此外,本研究还发现,预后不良组血清endocan水平显著高于预后良好组,且血清endocan是RRI患儿预后的独立影响因素。由此推测, endocan可能通过介导内皮功能和白细胞黏附等过程影响RRI患儿的预后[17]。彭贵鑫[18]发现,血浆endocan有助于慢性阻塞性肺疾病的病情评估和预后预测。本研究ROC曲线显示,血清endocan预测RRI患儿预后不良的AUC为0.777, 即能够在一定程度上对RRI患儿近期预后进行区分预测。
IL-1α为IL-1家族成员之一,是由活化的上皮细胞、巨噬细胞等细胞产生的促炎细胞因子,在机体细胞免疫和炎症反应中发挥重要作用。人类IL-1α编码基因位于2号染色体上[19-20]。佳丽娟等[21]报道,肺结核分歧杆菌感染患者的血清IL-1α水平升高,这可能由病原菌感染后巨噬细胞分泌IL-1α所致。RENIERIS G等[22]研究表明, IL-1α在新型冠状病毒感染中介导组织特异性炎症。本研究发现, RRI患儿血清IL-1α水平显著高于健康儿童,提示RRI患儿体内存在炎症反应; 重度组RRI患儿血清IL-1α水平显著高于中度组,且中度组显著高于轻度组,提示RRI患儿的病情越严重,炎症反应越强。结合Spearman相关性分析结果推测, IL-1α通过介导炎症反应与RRI严重程度密切相关。此外,本研究还发现,预后不良组患儿的血清IL-1α水平显著高于预后良好组,提示RRI患儿的预后与炎症反应强度存在一定关联。ROC曲线分析结果显示,血清IL-1α预测RRI患儿预后不良的AUC为0.795, 敏感度为68.18%, 特异度为82.81%, 提示血清IL-1α具有一定预测价值,当患儿血清IL-1α高于9.63 pg/mL时,预后不良的概率较高。
YKL-40是一种分子量约为40 kDa的分泌型糖蛋白,可由多种细胞分泌,参与组织重塑和炎症反应,其编码基因位于1号染色体。研究[23-24]表明, YKL-40与肺炎、糖尿病肾病、脑梗死等多种疾病的发展密切相关。缪治永等[25]研究发现,抑制YKL-40表达能够减轻Ⅱ型肺泡上皮细胞的炎症反应,为肺部疾病的治疗提供了新思路。本研究结果显示, RRI患儿血清YKL-40水平显著高于健康儿童,表明YKL-40参与了RRI的发生。进一步分析发现,血清YKL-40水平与RRI患儿病情严重程度密切相关,推测YKL-40可能通过介导呼吸系统炎症反应和氧化应激损伤影响其病情程度。此外,预后不良组RRI患儿的血清YKL-40水平显著高于预后良好组,且RRI患儿血清YKL-40水平越高,短期内预后不良的概率越高。郝淑娟等[26]发现,血清YKL-40是评估新生儿肺炎病情的重要指标之一,且其对预后的预测价值较高。本研究ROC曲线分析结果显示,血清YKL-40预测RRI患儿预后的AUC为0.787,敏感度和特异度分别为72.73%和82.81%, 表明血清YKL-40对RRI患儿预后具有一定预测价值。本研究进一步分析血清endocan、IL-1α、YKL-40三者联合对RRI患儿预后的预测价值,发现联合预测的敏感度高达90.91%, 且AUC(0.925)显著大于各指标单独预测的AUC, 表明联合预测的价值更高,可为临床治疗和护理工作提供指导。
综上所述,血清endocan、IL-1α、YKL-40水平均与RRI患儿的病情严重程度和预后密切相关,三者联合检测对RRI患儿预后具有良好的预测价值,可为病情评估和预后预测提供参考。
-
[1] GUO M F, ZHANG H Y, LI Y H, et al. Fasudil inhibits the activation of microglia and astrocytes of transgenic Alzheimer′s disease mice via the downregulation of TLR4/Myd88/NF-κB pathway[J]. J Neuroimmunol, 2020, 346: 577284. doi: 10.1016/j.jneuroim.2020.577284
[2] MEI B, LI J, ZUO Z Y. Dexmedetomidine attenuates Sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor[J]. Brain Behav Immun, 2021, 91: 296-314. doi: 10.1016/j.bbi.2020.10.008
[3] MARINO LEE S, HUDOBENKO J, MCCULLOUGH L D, et al. Microglia depletion increase brain injury after acute ischemic stroke in aged mice[J]. Exp Neurol, 2021, 336: 113530. doi: 10.1016/j.expneurol.2020.113530
[4] XIONG X Y, LIU L, YANG Q W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke[J]. Prog Neurobiol, 2016, 142: 23-44. doi: 10.1016/j.pneurobio.2016.05.001
[5] YE Y Z, JIN T, ZHANG X, et al. Meisoindigo protects against focal cerebral ischemia-reperfusion injury by inhibiting NLRP3 inflammasome activation and regulating microglia/macrophage polarization via TLR4/NF-κB signaling pathway[J]. Front Cell Neurosci, 2019, 13: 553. doi: 10.3389/fncel.2019.00553
[6] IRING A, TÓTH A, BARANYI M, et al. The dualistic role of the purinergic P2Y12-receptor in an in vivo model of Parkinson's disease: signalling pathway and novel therapeutic targets[J]. Pharmacol Res, 2022, 176: 106045. doi: 10.1016/j.phrs.2021.106045
[7] LIU Y L, WU C F, HOU Z J, et al. Pseudoginsenoside-F11 accelerates microglial phagocytosis of myelin debris and attenuates cerebral ischemic injury through complement receptor 3[J]. Neuroscience, 2020, 426: 33-49. doi: 10.1016/j.neuroscience.2019.11.010
[8] ZANDI S, NAKAO S, CHUN K H, et al. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration[J]. Cell Rep, 2015, 10(7): 1173-1186. doi: 10.1016/j.celrep.2015.01.050
[9] VAROL C, MILDNER A, JUNG S. Macrophages: development and tissue specialization[J]. Annu Rev Immunol, 2015, 33: 643-675. doi: 10.1146/annurev-immunol-032414-112220
[10] SAIJO K, GLASS C K. Microglial cell origin and phenotypes in health and disease[J]. Nat Rev Immunol, 2011, 11(11): 775-787. doi: 10.1038/nri3086
[11] WANG J. Preclinical and clinical research on inflammation after intracerebral hemorrhage[J]. Prog Neurobiol, 2010, 92(4): 463-477. doi: 10.1016/j.pneurobio.2010.08.001
[12] GINHOUX F, GRETER M, LEBOEUF M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages[J]. Science, 2010, 330(6005): 841-845. doi: 10.1126/science.1194637
[13] CHIOT A, ZAÏDI S, ILTIS C, et al. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival[J]. Nat Neurosci, 2020, 23(11): 1339-1351. doi: 10.1038/s41593-020-00718-z
[14] XU Y, CUI K X, LI J, et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway[J]. J Pineal Res, 2020, 69(1): e12660.
[15] 庄欣琪, 王玉尊, 王瑶琪, 等. 氢对LPS致BV-2小胶质细胞炎症反应的影响及自噬在其中的作用[J]. 中华麻醉学杂志, 2020, 40(3): 350-354. doi: 10.3760/cma.j.cn131073.20190726.00324 [16] HU X M, LEAK R K, SHI Y J, et al. Microglial and macrophage polarization—new prospects for brain repair[J]. Nat Rev Neurol, 2015, 11(1): 56-64. doi: 10.1038/nrneurol.2014.207
[17] 刘太聪, 史永强, 张海鸿. 间充质干细胞在神经病理性疼痛中的作用及机制研究[J]. 实用临床医药杂志, 2022, 26(6): 113-117. doi: 10.7619/jcmp.20213816 [18] ZHANG L J, ZHANG J Q, YOU Z L. Switching of the microglial activation phenotype is a possible treatment for depression disorder[J]. Front Cell Neurosci, 2018, 12: 306.
[19] LARSON-CASEY J L, VAID M, GU L L, et al. Increased flux through the mevalonate pathway mediates fibrotic repair without injury[J]. J Clin Invest, 2019, 129(11): 4962-4978. doi: 10.1172/JCI127959
[20] ALVES A, DIEL L, RAMOS G, et al. Tumor microenvironment and Oral Squamous Cell Carcinoma: a crosstalk between the inflammatory state and tumor cell migration[J]. Oral Oncol, 2021, 112: 105038. doi: 10.1016/j.oraloncology.2020.105038
[21] ZHENG Y, HE R Y, WANG P, et al. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization[J]. Biomater Sci, 2019, 7(5): 2037-2049. doi: 10.1039/C8BM01449C
[22] TANG Y Y, HE Y, ZHANG P, et al. LncRNAs regulate the cytoskeleton and related Rho/ROCK signaling in cancer metastasis[J]. Mol Cancer, 2018, 17(1): 77. doi: 10.1186/s12943-018-0825-x
[23] HEMKEMEYER S A, VOLLMER V, SCHWARZ V, et al. Local Myo9b RhoGAP activity regulates cell motility[J]. J Biol Chem, 2021, 296: 100136. doi: 10.1074/jbc.RA120.013623
[24] GARCÍA-MARISCAL A, LI H, PEDERSEN E, et al. Loss of RhoA promotes skin tumor formation and invasion by upregulation of RhoB[J]. Oncogene, 2018, 37(7): 847-860. doi: 10.1038/onc.2017.333
[25] ALKASALIAS T, ALEXEYENKO A, HENNIG K, et al. RhoA knockout fibroblasts lose tumor-inhibitory capacity in vitro and promote tumor growth in vivo[J]. Proc Natl Acad Sci USA, 2017, 114(8): E1413-E1421.
[26] LAI A Y, MCLAURIN J. Rho-associated protein kinases as therapeutic targets for both vascular and parenchymal pathologies in Alzheimer's disease[J]. J Neurochem, 2018, 144(5): 659-668. doi: 10.1111/jnc.14130
[27] LU W Z, WEN J Y, CHEN Z W. Distinct roles of ROCK1 and ROCK2 on the cerebral ischemia injury and subsequently neurodegenerative changes[J]. Pharmacology, 2020, 105(1/2): 3-8.
[28] SZASZ T, WEBB R C. Rho-mancing to sensitize calcium signaling for contraction in the vasculature: role of rho kinase[J]. Adv Pharmacol, 2017, 78: 303-322.
[29] KANG H, YANG B G, ZHANG K Y, et al. Immunoregulation of macrophages by dynamic ligand presentation via ligand-cation coordination[J]. Nat Commun, 2019, 10(1): 1696. doi: 10.1038/s41467-019-09733-6
[30] KANG H, WONG S H D, PAN Q, et al. Anisotropic ligand nanogeometry modulates the adhesion and polarization state of macrophages[J]. Nano Lett, 2019, 19(3): 1963-1975. doi: 10.1021/acs.nanolett.8b05150
[31] BORRAJO A, RODRIGUEZ-PEREZ A I, VILLAR-CHEDA B, et al. Inhibition of the microglial response is essential for the neuroprotective effects of Rho-kinase inhibitors on MPTP-induced dopaminergic cell death[J]. Neuropharmacology, 2014, 85: 1-8. doi: 10.1016/j.neuropharm.2014.05.021
[32] XUE H, ZHANG Y H, GAO Q S, et al. Sevoflurane post-conditioning ameliorates neuronal deficits and axon demyelination after neonatal hypoxic ischemic brain injury: role of microglia/macrophage[J]. Cell Mol Neurobiol, 2021, 41(8): 1801-1816. doi: 10.1007/s10571-020-00949-5
[33] YU J G, OGAWA K, TOKINAGA Y, et al. Sevoflurane inhibits guanosine 5′-[gamma-thio]triphosphate-stimulated, Rho/Rho-kinase-mediated contraction of isolated rat aortic smooth muscle[J]. Anesthesiology, 2003, 99(3): 646-651. doi: 10.1097/00000542-200309000-00020
[34] ZHANG H F, LI Y H, YU J Z, et al. Rho kinase inhibitor fasudil regulates microglia polarization and function[J]. Neuroimmunomodulation, 2013, 20(6): 313-322. doi: 10.1159/000351221
[35] WEI H X, YAO P S, CHEN P P, et al. Neuronal EphA4 regulates OGD/R-induced apoptosis by promoting alternative activation of microglia[J]. Inflammation, 2019, 42(2): 572-585. doi: 10.1007/s10753-018-0914-4
[36] JING F, ZHANG Y X, LONG T, et al. P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine[J]. J Neuroinflammation, 2019, 16(1): 217. doi: 10.1186/s12974-019-1603-4
[37] CHEN C, LI Y H, ZHANG Q, et al. Fasudil regulates T cell responses through polarization of BV-2 cells in mice experimental autoimmune encephalomyelitis[J]. Acta Pharmacol Sin, 2014, 35(11): 1428-1438. doi: 10.1038/aps.2014.68
[38] ZHANG X X, YE P, WANG D D, et al. Involvement of RhoA/ROCK signaling in aβ-induced chemotaxis, cytotoxicity and inflammatory response of microglial BV2 cells[J]. Cell Mol Neurobiol, 2019, 39(5): 637-650. doi: 10.1007/s10571-019-00668-6
[39] LU E M, WANG Q, LI S C, et al. Profilin 1 knockdown prevents ischemic brain damage by promoting M2 microglial polarization associated with the RhoA/ROCK pathway[J]. J Neurosci Res, 2020, 98(6): 1198-1212. doi: 10.1002/jnr.24607
[40] REFOLO V, STEFANOVA N. Neuroinflammation and glial phenotypic changes in alpha-synucleinopathies[J]. Front Cell Neurosci, 2019, 13: 263.
[41] SACKMANN V, ANSELL A, SACKMANN C, et al. Anti-inflammatory (M2) macrophage media reduce transmission of oligomeric amyloid beta in differentiated SH-SY5Y cells[J]. Neurobiol Aging, 2017, 60: 173-182. doi: 10.1016/j.neurobiolaging.2017.08.022
[42] 张琳, 张伟, 张加强, 等. 利多卡因对大鼠内毒素性肺损伤时Rho/ROCK信号通路的影响[J]. 中华麻醉学杂志, 2019, 39(1): 109-112. doi: 10.3760/cma.j.issn.0254-1416.2019.01.028 [43] SCHEIBLICH H, BICKER G. Regulation of microglial phagocytosis by RhoA/ROCK-inhibiting drugs[J]. Cell Mol Neurobiol, 2017, 37(3): 461-473. doi: 10.1007/s10571-016-0379-7
[44] PENG F, LU L Y, WEI F, et al. The onjisaponin B metabolite tenuifolin ameliorates dopaminergic neurodegeneration in a mouse model of Parkinson′s disease[J]. Neuroreport, 2020, 31(6): 456-465. doi: 10.1097/WNR.0000000000001428
[45] WONG S S C, LEE U M, WANG X M, et al. Role of DLC2 and RhoA/ROCK pathway in formalin induced inflammatory pain in mice[J]. Neurosci Lett, 2019, 709: 134379. doi: 10.1016/j.neulet.2019.134379
[46] LEE J, VILLARREAL O D, CHEN X R, et al. QUAKING regulates microexon alternative splicing of the rho GTPase pathway and controls microglia homeostasis[J]. Cell Rep, 2020, 33(13): 108560. doi: 10.1016/j.celrep.2020.108560
[47] VILLAR-CHEDA B, DOMINGUEZ-MEIJIDE A, JOGLAR B, et al. Involvement of microglial RhoA/Rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors[J]. Neurobiol Dis, 2012, 47(2): 268-279. doi: 10.1016/j.nbd.2012.04.010
[48] HAN X N, LAN X, LI Q, et al. Inhibition of prostaglandin E2 receptor EP3 mitigates thrombin-induced brain injury[J]. J Cereb Blood Flow Metab, 2016, 36(6): 1059-1074. doi: 10.1177/0271678X15606462
[49] ZHU M M, LIN J H, QING P, et al. Manual acupuncture relieves microglia-mediated neuroinflammation in a rat model of traumatic brain injury by inhibiting the RhoA/ROCK2 pathway[J]. Acupunct Med, 2020, 38(6): 426-434. doi: 10.1177/0964528420912248
[50] QIAN Z Y, CHEN H T, XIA M J, et al. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury[J]. Int J Biol Sci, 2022, 18(4): 1328-1346. doi: 10.7150/ijbs.68974
[51] KISHIMA K, TACHIBANA T, YAMANAKA H, et al. Role of Rho-associated coiled-coil containing protein kinase in the spinal cord injury induced neuropathic pain[J]. Spine J, 2021, 21(2): 343-351. doi: 10.1016/j.spinee.2020.08.011
[52] TATSUMI E, YAMANAKA H, KOBAYASHI K, et al. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain[J]. Glia, 2015, 63(2): 216-228. doi: 10.1002/glia.22745
[53] MUESSEL M J, HARRY G J, ARMSTRONG D L, et al. SDF-1α and LPA modulate microglia potassium channels through rho gtpases to regulate cell morphology[J]. Glia, 2013, 61(10): 1620-1628. doi: 10.1002/glia.22543
[54] MOON M Y, KIM H J, LI Y, et al. Involvement of small GTPase RhoA in the regulation of superoxide production in BV2 cells in response to fibrillar Aβ peptides[J]. Cell Signal, 2013, 25(9): 1861-1869. doi: 10.1016/j.cellsig.2013.05.023
[55] DE CARIS M G, GRIECO M, MAGGI E, et al. Blueberry counteracts BV-2 microglia morphological and functional switch after LPS challenge[J]. Nutrients, 2020, 12(6): 1830. doi: 10.3390/nu12061830
[56] KOCH J C, KUTTLER J, MAASS F, et al. Compassionate use of the ROCK inhibitor fasudil in three patients with amyotrophic lateral sclerosis[J]. Front Neurol, 2020, 11: 173. doi: 10.3389/fneur.2020.00173
-
期刊类型引用(5)
1. 胡晓敏,孔晓明,孙艳,张丽,吕思文,谢朋宇,郭雨. 共病2型糖尿病的老年期抑郁障碍患者血糖波动及与脂代谢特征的相关性研究. 临床医学研究与实践. 2024(16): 64-67 .  百度学术
百度学术
2. 朱林美. 2型糖尿病患者行常规心电图检查的意义. 名医. 2024(03): 93-95 .  百度学术
百度学术
3. 王惠,吴茜,李晴,邱彩玉,周晶晶. 空腹血糖、脂代谢指标与无症状高血压人群心电图ST-T改变关系及其诊断价值研究. 陕西医学杂志. 2023(09): 1196-1199 .  百度学术
百度学术
4. 陈晓琪. 86例高龄糖尿病患者的临床情况分析. 糖尿病新世界. 2022(06): 42-45 .  百度学术
百度学术
5. 魏峰,张玉顺,王星烨,霍建华. 高糖与胰岛素对人诱导多潜能干细胞来源的心肌细胞电生理特性的影响. 中南大学学报(医学版). 2022(05): 610-618 .  百度学术
百度学术
其他类型引用(1)




 下载:
下载:

 苏公网安备 32100302010246号
苏公网安备 32100302010246号
