Construction and validation of prognostic risk model for patients with hepatocellular carcinoma based on bioinformatics analysis
-
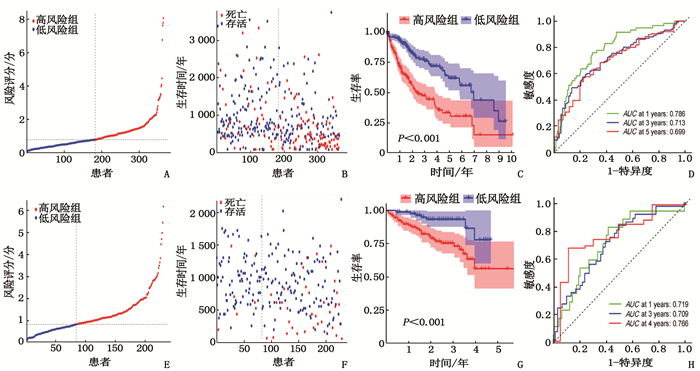
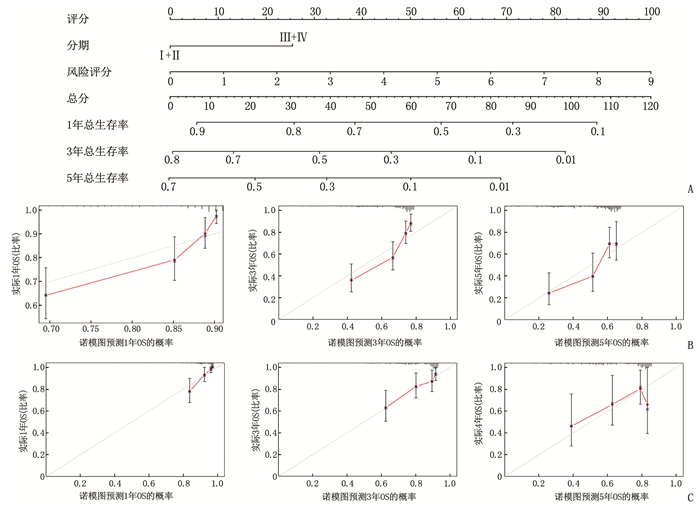
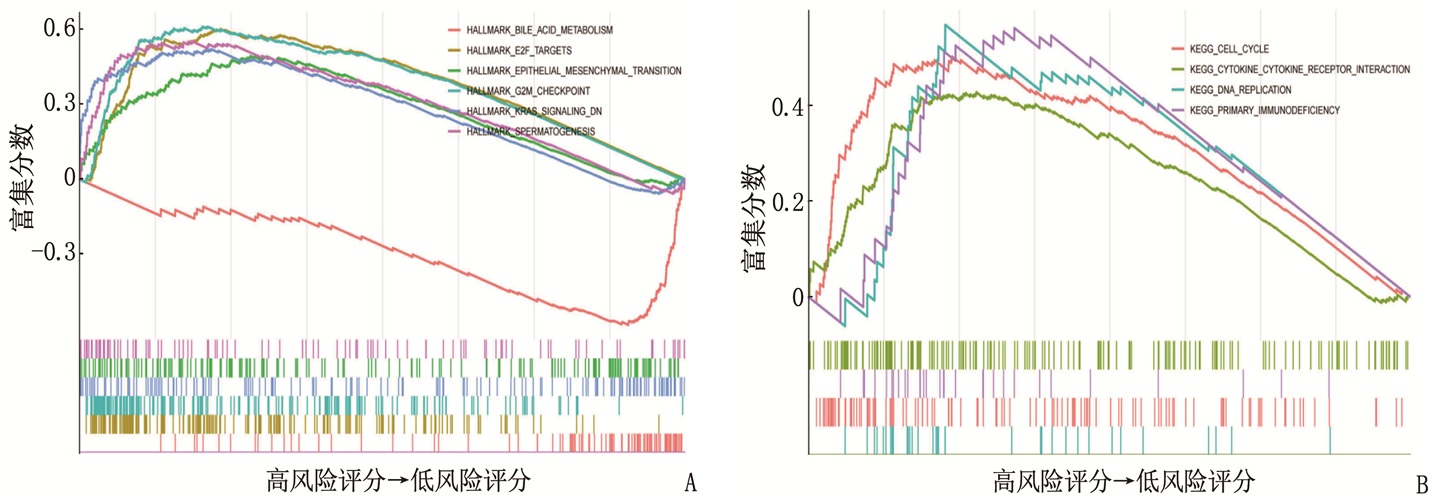
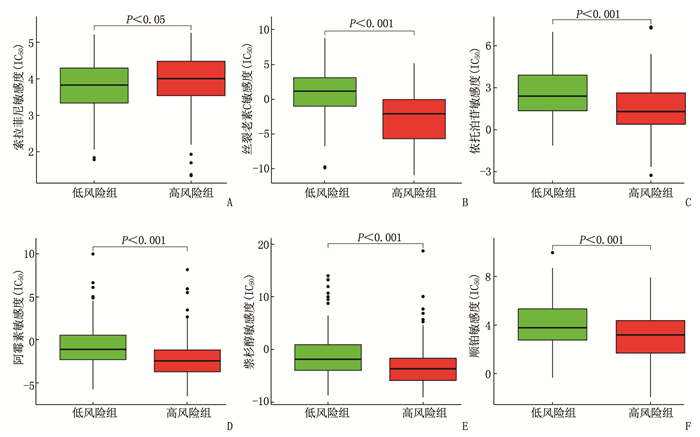
摘要:目的 利用公共数据库构建用于临床治疗肝细胞癌(HCC)的预后风险模型。方法 分别从癌症基因组图谱(TCGA)和国际癌症基因组联盟(ICGC)下载HCC以及癌旁正常组织的mRNA表达数据及临床信息。在TCGA队列中筛选与总生存期(OS)相关的差异表达基因(DEGs), 从中抽取2个或3个mRNAs构成一个组合, 对所有组合进行Cox风险比例回归模型构建。通过受试者工作特征(ROC)曲线的曲线下面积(AUC)确定最优基因组合,并进行基于ICGC队列的外部验证; 以TCGA队列的风险评分中位值将患者分为高风险组与低风险组,进行基因集富集分析(GSEA), 并通过pRRophetic R软件包预测HCC患者使用索拉非尼、丝裂霉素、依托泊苷、阿霉素、紫杉醇和顺铂的相对半抑制浓度(IC50)。结果 该预后风险模型预测TCGA队列的1、3、5年OS的ROC的AUC分别是0.786、0.713、0.699, 预测ICGC队列的1、3、4年OS的ROC的AUC分别为0.719、0.709、0.766。GSEA表明高风险组患者细胞周期相关通路被激活,胆汁酸代谢被抑制。索拉非尼在低风险组的IC50低于高风险组,而细胞周期相关化疗药物在低风险组的IC50高于高风险组,差异均有统计学意义(P < 0.05)。结论 本研究建立并验证了HCC预后风险模型,为HCC患者个体化诊疗方案的制订提供参考依据。Abstract:Objective To construct a prognostic risk model for clinical treatment of hepatocellular carcinoma (HCC) based on public databases.Methods The mRNA expression data and clinical information of HCC and adjacent normal tissues were downloaded from The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC). Differentially expressed genes (DEGs) related to overall survival (OS) were screened in the TCGA cohort, 2 or 3 mRNAs were selected to form a combination, and Cox risk proportional regression model was constructed for all combinations. The optimal gene combination was determined by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve, and external validation based on ICGC cohort was carried out. The patients were divided into high-risk group and low-risk group according to the median risk score of TCGA cohort, gene set enrichment analysis (GSEA) was performed, and the relative half-inhibitory concentrations (IC50) of sorafenib, mitomycin, etoposide, adriamycin, paclitaxel and cisplatin in HCC patients were predicted by pRRophetic R software package.Results For this prognostic risk model, the AUC of the ROC curve for predicting 1-, 3- and 5-year OS in the TCGA cohort were 0.786, 0.713 and 0.699, respectively, and the AUC of the ROC curve for predicting 1-, 3- and 4-year OS in the ICGC cohort were 0.719, 0.709 and 0.766, respectively. GSEA revealed that cell cycle related pathways were activated and bile acid metabolism was inhibited in the high-risk group. The IC50 of sorafenib in the low-risk group was significantly lower than that in the high-risk group, while the IC50 of cell cycle related chemotherapy drugs in the low-risk group was significantly higher than that in the high-risk group (P < 0.05).Conclusion This study establishes and verifies the prognostic risk model for HCC, and provides a reference for the formulation of individualized diagnosis and treatment plan for HCC patients.
-
成人足舟骨坏死(Müller-Weiss病)是一种罕见的足部疾病,主要发生在40~60岁中年女性中。早在1925年SCHMIDT描述了一个多腺功能不全患者,结合足舟骨形态,认为是足舟骨受力压缩导致。1927年WALTHER MVLLER提出该病由先天性缺陷导致,随后KONRAD WEISS也阐释了相似情况,认为真正病因为足舟骨坏死,故命名为Müller-Weiss病。该病通常表现为双侧分布,患者足部疼痛,并伴有足舟骨畸形,出现足舟骨进行性坏死[1]。由于足舟骨外侧压缩、碎裂,距骨头向外半脱位,表现为“逗号状”或“沙漏状”症状。最终结果为内侧足弓代偿性变低,导致平足及“距舟楔关节”形成。关于Müller-Weiss病原因现在仍然存在争议,且易误诊及漏诊。该病保守治疗无效的前提下,首选的还是手术治疗。MACEIRA E等[2]通过总结分析最大的Müller-Weiss病序列,认为足舟状骨的延迟骨化和其异常的应力是导致Müller-Weiss病的重要原因。本文就Müller-Weiss病的临床特征、影像学特点、鉴别诊断和治疗方法等进行综述研究。
1. 足舟骨的解剖
足舟骨是内侧柱稳定的基础,内侧纵弓及横弓因足舟骨而完整。足舟骨足底侧血供是由足底内侧动脉供应,背侧和外侧血液供应由足背动脉供应。由于这些周边血运交叉,在足舟骨的中央区域。若血液灌注不足,且承受距骨、内侧楔骨对其的挤压应力,足舟骨区剪切应力最大,那极易发生应力骨折以及缺血坏死。这一解剖特性随着患者年龄的增长而增加风险,独特的生理解剖决定了舟骨区应力性骨折和骨坏死发生率最高[3]。此外包括糖尿病及红斑狼疮在内的全身性疾病和药物及过量饮酒也会引起足舟骨的坏死[4]。
2. 临床表现
Müller-Weiss病早期表现为负重时中足背侧疼痛或者后足疼痛,随着病情发展,导致足底畸形及致残性疼痛[5]。足背内侧常伴随压痛,足舟骨有突出,纵弓下沉,导致扁平足畸形,后足内翻,距下关节活动度减少[6]。由于后足立线改变导致胫骨被迫外旋,膝关节生物力学发生变化,部分患者会出现膝关节行走时疼痛。
3. 影像学检查
数字化X线摄影(DR)及计算机断层扫描(CT)可以发现足舟骨外部碎裂以及中足部关节炎的形成。负重CT和负重位X线对于诊断Müller-Weiss有重要的影像学价值,核磁共振成像(MRI)以及核素扫描对于发现中足部炎症信号及关节退变有一定意义。Müller-Weiss病早期X片检查可见足舟状骨密度增高伴外侧压缩、舟骨斜裂及距骨头侧向移位。足舟骨随着外侧压缩,足背以及内侧逐渐突出,整个舟骨可一分为二。MACEIRA E等[2]根据患足在负重侧位X线上,距骨轴线与第1跖骨轴线的交角(M-T角)和足畸形的程度,将Müller-Weiss病分为5期。Ⅰ期: X线显示轻微改变或正常, CT和骨扫描表现不显著,核磁共振可出现骨水肿表现; Ⅱ期: 距下关节内翻,形成高弓足表现,距骨轴线与第1跖骨轴线的交角指向足背,距骨头半脱位,跗骨管和跗骨窦呈孔洞样表现,距骨头与跟骨重叠减小; Ⅲ期: 足弓下沉,后足渐内翻,足舟骨出现压缩碎裂,距骨轴线与第1跖骨轴线的交角接近0°,距骨楔骨间距显著减小; Ⅳ期: 后足进一步内翻,足弓显著下沉,足舟骨压缩加剧,距骨轴线与第1跖骨轴线的交角指向足底; Ⅴ期: 足舟骨完全脱出,导致距楔关节形成。但是长期随访后的研究[7]指出,患者症状与分期并不完全吻合,分期系统可有助于理解该病综合征,但是不同分期过渡过程中,畸形程度未必能清晰看出,故临床症状不一定与影像学检查完全吻合。
4. 鉴别诊断
足舟骨缺血性坏死(KÖhler病)通常是单侧的,患者发病年龄3~7岁,症状轻微,有男性先兆。Müller-Weiss病通常是双侧的,有特征性疼痛的临床过程和进行性畸形。而KÖhler病则是一种自限性疾病,愈后较良好[8]。Müller-Weiss病与夏科氏关节病足的区别也比较明确,夏科氏足由于周围神经病变导致痛觉减低,而Müller-Weiss病有个较长的渐进性疼痛过程。该病与足舟骨继发性骨坏死相比较,足舟骨继发性骨坏死与外伤、肾功能衰竭、类风湿性关节炎有关。
5. 治疗方法
5.1 保守治疗方法
Müller-Weiss病初期以保守治疗为主,包括口服消炎药、定制矫形器,需要石膏固定,减少活动。定制矫形器是常用手段,可以有效减少疼痛,通过限制患者活动,使用内增强的足底支撑鞋垫,增强内侧结构,减少足舟骨在行走过程中受到的周围骨骼的压迫。急性疼痛非常剧烈时,应先用石膏靴固定5~6周再行手术,会有较好的预期效果[9]。
5.2 手术治疗
对于MACEIRA分期Ⅲ期及以上者,主张手术治疗。关节融合术主要根据病变所累及的关节,以减少疼痛,避免平足畸形发生,恢复内侧柱长度。手术方法包括距舟关节融合、距舟楔关节融合和三关节融合等[10]。
5.2.1 距舟关节融合
距舟关节融合适用于病变局限在距舟关节,且病变较轻的患者。距舟关节融合特点在于复位距舟关节良好融合,纠正距骨外移、外旋及跖屈,纠正舟骨的内旋。在足正侧位上恢复距骨-第1跖骨轴线,改善距骨的外移及外旋,在一定程度上纠正后足的内翻畸形,恢复后足的力线。利用髂骨植入恢复中足支撑作用,降低距舟关节外侧剪切力,临床回访效果较理想[11]。屈福锋等[12]认为单纯距舟关节融合有一定的疗效,结合跟骨截骨优势更加显著。
5.2.2 距舟楔关节融合
对于病变发展到舟楔关节合并距周关节病变时,有必要行距舟楔融合。FERNÁNDEZ DE RETANA P等[13]通过使用自体髂骨植骨,替代缺损的舟骨,不使用钢板固定。TAN A等[14]对1例患者行足舟骨切除术,用异体股骨头塑形替代舟骨,以自体髂骨植骨,钢板跨距舟楔关节固定。术后随访患者无不适主诉。该方法足弓得到很好的恢复,融合效果佳。
5.2.3 三关节融合
对于晚期患者(MACEIRA分期Ⅳ~Ⅴ期和部分Ⅲ期患者),尤其是出现显著形态学改变及足部骨关节炎时,传统手术方式是切开三关节融合术,研究[14]表明,三关节融合稳定性高于单关节融合。三关节融合术可以提供内侧和外侧稳定性,从而达到良好的固定效果。踝关节镜技术的发展对治疗Müller-Weiss病发挥很大作用[15-16]。LUI T H[17]通过对6只患足行关节镜下三关节融合,镜下探查显示1例出现跖骨下退变, 4例患有足底滑膜炎, 5例有跟骰关节骨性关节炎, 2例跟骰关节滑膜炎。所有患足均显著踝关节退化。术后患者满意,平均固定融合时间为21周(16~22周)。关节镜对于治疗Müller-Weiss病有着软组织损伤小,融合稳定性高的优势,对治疗关节早期炎性病变有较好疗效。此外钻孔可以释放骨内压力,改善微循环及代谢,对于缓解疼痛有着一定的疗效[18-19]。
6. 总结
现阶段人们对Müller-Weiss病的认识不断深入,诊断和治疗技术不断提升[20-21]。患者影像学表现的轻重未必与实际症状相符,因此在治疗和诊断中要因人而异和因病而异,治疗方案的选择要综合影像学报告及临床体征,并根据不同手术方案各自的优缺点不断完善手术策略。
-
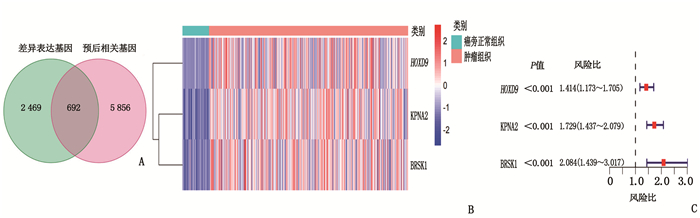
表 1 HCC患者的临床病理资料[n(%)]
临床资料 TCGA队列
(n=365)ICGC队列
(n=231)年龄/岁 61(16, 90) 69(31, 89) 性别 女 119(32.6) 61(26.4) 男 246(67.4) 170(72.6) 肿瘤分级 Ⅰ级 55(15.1) — Ⅱ级 175(47.9) — Ⅲ级 118(32.3) — Ⅳ级 12(3.3) — 未知 5(1.4) — 肿瘤分期 Ⅰ期 170(46.6) 36(15.6) Ⅱ期 84(23.0) 105(45.5) Ⅲ期 83(22.7) 71(30.7) Ⅳ期 4(1.1) 19(8.2) 未知 24(6.6) 0 血管浸润 有 106(29.0) — 无 205(56.2) — 未知 54(14.8) — 甲胎蛋白 ≤200 ng/mL 201(55.1) — > 200 ng/mL 75(20.5) — 未知 89(24.4) — 年龄以中位数(最小值,最大值)表示。 表 2 风险评分与HCC患者临床病理资料的相关性
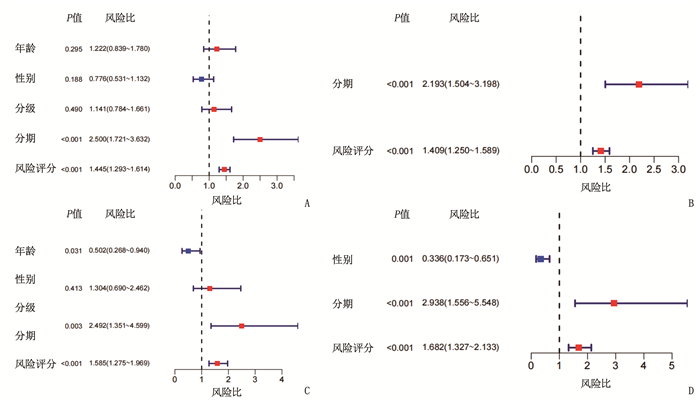
临床资料 TCGA队列 ICGC队列 高风险组 低风险组 P 高风险组 低风险组 P 性别 0.80 1.00 女性 58 61 22 39 男性 125 121 62 108 年龄 0.80 0.42 < 60岁 81 84 29 60 ≥60岁 102 98 55 87 肿瘤分级 < 0.05 - 1、2级 134 96 - - 3、4级 47 83 - - 肿瘤分期 < 0.05 < 0.05 Ⅰ、Ⅱ期 141 113 63 78 Ⅲ、Ⅳ期 30 57 21 69 血管浸润 < 0.05 - 无 124 81 - - 有 44 62 - - 甲胎蛋白 < 0.05 - ≤200 ng/mL 122 79 - - >200 ng/mL 27 48 - - -
[1] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. doi: 10.1056/NEJMra1713263
[2] YANG J D, HAINAUT P, GORES G J, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(10): 589-604. doi: 10.1038/s41575-019-0186-y
[3] TORRE L A, BRAY F, SIEGEL R L, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. doi: 10.3322/caac.21262
[4] BROWN Z J, GRETEN T F, HEINRICH B. Adjuvant treatment of hepatocellular carcinoma: prospect of immunotherapy[J]. Hepatology, 2019, 70(4): 1437-1442. doi: 10.1002/hep.30633
[5] HUANG W T, SKANDERUP A J, LEE C G. Advances in genomic hepatocellular carcinoma research[J]. Gigascience, 2018, 7(11): 1-22. http://www.onacademic.com/detail/journal_1000041617495499_6d02.html
[6] DOMINGUEZ D A, WANG X W. Impact of next-generation sequencing on outcomes in hepatocellular carcinoma: how precise are we really[J]. J Hepatocell Carcinoma, 2020, 7: 33-37. doi: 10.2147/JHC.S217948
[7] CARUSO S, O'BRIEN D R, CLEARY S P, et al. Genetics of hepatocellular carcinoma: approaches to explore molecular diversity[J]. Hepatology, 2021, 73(Suppl 1): 14-26. http://www.researchgate.net/publication/348322949_Genetics_of_Hepatocellular_Carcinoma_Approaches_to_Explore_Molecular_Diversity
[8] GEELEHER P, COX N, HUANG R S. pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels[J]. PLoS One, 2014, 9(9): e107468. doi: 10.1371/journal.pone.0107468
[9] PAN Y S, CHEN H R, YU J. Biomarkers in hepatocellular carcinoma: current status and future perspectives[J]. Biomedicines, 2020, 8(12): 576. doi: 10.3390/biomedicines8120576
[10] OURA K, MORISHITA A, MASAKI T. Molecular and functional roles of microRNAs in the progression of hepatocellular carcinoma-A review[J]. Int J Mol Sci, 2020, 21(21): 8362. doi: 10.3390/ijms21218362
[11] KIM E, VIATOUR P. Hepatocellular carcinoma: old friends and new tricks[J]. Exp Mol Med, 2020, 52(12): 1898-1907. doi: 10.1038/s12276-020-00527-1
[12] MUINAO T, DEKA BORUAH H P, PAL M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer[J]. Heliyon, 2019, 5(12): e02826. doi: 10.1016/j.heliyon.2019.e02826
[13] FOUNTZILAS C, KAKLAMANI V G. Multi-gene panel testing in breast cancer management[J]. Cancer Treat Res, 2018, 173: 121-140.
[14] YANG Z C, ZI Q, XU K, et al. Development of a macrophages-related 4-gene signature and nomogram for the overall survival prediction of hepatocellular carcinoma based on WGCNA and LASSO algorithm[J]. Int Immunopharmacol, 2021, 90: 107238. doi: 10.1016/j.intimp.2020.107238
[15] LIU G M, XIE W X, ZHANG C Y, et al. Identification of a four-gene metabolic signature predicting overall survival for hepatocellular carcinoma[J]. J Cell Physiol, 2020, 235(2): 1624-1636. doi: 10.1002/jcp.29081
[16] LIN P, HE R Q, DANG Y W, et al. An autophagy-related gene expression signature for survival prediction in multiple cohorts of hepatocellular carcinoma patients[J]. Oncotarget, 2018, 9(25): 17368-17395. doi: 10.18632/oncotarget.24089
[17] LI G X, XU W Q, ZHANG L, et al. Development and validation of a CIMP-associated prognostic model for hepatocellular carcinoma[J]. EBioMedicine, 2019, 47: 128-141. doi: 10.1016/j.ebiom.2019.08.064
[18] YU J, WU X L, LV M, et al. A model for predicting prognosis in patients with esophageal squamous cell carcinoma based on joint representation learning[J]. Oncol Lett, 2020, 20(6): 387. http://www.ncbi.nlm.nih.gov/pubmed/33193847
[19] YANG Y J, WANG C Y, WEI N D, et al. Identification of prognostic chromatin-remodeling genes in clear cell renal cell carcinoma[J]. Aging (Albany NY), 2020, 12(24): 25614-25642.
[20] HONG W F, LIANG L, GU Y J, et al. Immune-related lncRNA to construct novel signature and predict the immune landscape of human hepatocellular carcinoma[J]. Mol Ther Nucleic Acids, 2020, 22: 937-947. doi: 10.1016/j.omtn.2020.10.002
[21] 陈懿, 李雪, 林文雅, 等. 自噬基因预测肝癌患者长期生存及通路分析[J]. 医学研究杂志, 2021, 50(1): 137-141. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYZ202101031.htm [22] 段万里, 任伟, 邓骞, 等. 基于TCGA数据库的肾癌自噬相关基因预后模型的建立与应用[J]. 现代泌尿外科杂志, 2020, 25(10): 870-875, 889. doi: 10.3969/j.issn.1009-8291.2020.10.003 [23] TABUSE M, OHTA S, OHASHI Y, et al. Functional analysis of HOXD9 in human gliomas and glioma cancer stem cells[J]. Mol Cancer, 2011, 10: 60. doi: 10.1186/1476-4598-10-60
[24] LONG J Y, ZHANG L, WAN X S, et al. A four-gene-based prognostic model predicts overall survival in patients with hepatocellular carcinoma[J]. J Cell Mol Med, 2018, 22(12): 5928-5938. doi: 10.1111/jcmm.13863
[25] LV X P, LI L L, LV L, et al. HOXD9 promotes epithelial-mesenchymal transition and cancer metastasis by ZEB1 regulation in hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2015, 34: 133. doi: 10.1186/s13046-015-0245-3
[26] CHRISTIANSEN A, DYRSKJOT L. The functional role of the novel biomarker karyopherin α 2 (KPNA2) in cancer[J]. Cancer Lett, 2013, 331(1): 18-23. doi: 10.1016/j.canlet.2012.12.013
[27] GUO X G, WANG Z H, ZHANG J N, et al. Upregulated KPNA2 promotes hepatocellular carcinoma progression and indicates prognostic significance across human cancer types[J]. Acta Biochim Biophys Sin (Shanghai), 2019, 51(3): 285-292. doi: 10.1093/abbs/gmz003
[28] JIANG P, TANG Y Q, HE L, et al. Aberrant expression of nuclear KPNA2 is correlated with early recurrence and poor prognosis in patients with small hepatocellular carcinoma after hepatectomy[J]. Med Oncol, 2014, 31(8): 1-7.
[29] KONIROVA J, OLTOVA J, CORLETT A, et al. Modulated DISP3/PTCHD2 expression influences neural stem cell fate decisions[J]. Sci Rep, 2017, 7: 41597. doi: 10.1038/srep41597
[30] XIE G X, WANG X N, HUANG F J, et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis[J]. Int J Cancer, 2016, 139(8): 1764-1775. doi: 10.1002/ijc.30219
[31] CHEN W B, OU M L, TANG D E, et al. Identification and validation of immune-related gene prognostic signature for hepatocellular carcinoma[J]. J Immunol Res, 2020, 2020: 5494858. http://qikan.cqvip.com/Qikan/Article/Detail?id=7106408960
[32] STACEY D, KAZLAUSKAS A. Regulation of Ras signaling by the cell cycle[J]. Curr Opin Genet Dev, 2002, 12(1): 44-46. doi: 10.1016/S0959-437X(01)00262-3
[33] MATSUDA Y. Molecular mechanism underlying the functional loss of cyclindependent kinase inhibitors p16 and p27 in hepatocellular carcinoma[J]. World J Gastroenterol, 2008, 14(11): 1734-1740. doi: 10.3748/wjg.14.1734
[34] MATSUDA Y, ICHIDA T. p16 and p27 are functionally correlated during the progress of hepatocarcinogenesis[J]. Med Mol Morphol, 2006, 39(4): 169-175. doi: 10.1007/s00795-006-0339-2
[35] GREENBAUM L E. Cell cycle regulation and hepatocarcinogenesis[J]. Cancer Biol Ther, 2004, 3(12): 1200-1207. doi: 10.4161/cbt.3.12.1392
-
期刊类型引用(2)
1. 吴雨桐,杨子飞,王龄磊,张超. Müller-Weiss病继发双膝关节退变一例并文献复习. 骨科. 2025(01): 64-67 .  百度学术
百度学术
2. 张宏斌,荣锦,张立峰,刘婷婷,关鹏飞,王禹,张奉琪. 跟骨截骨配合距舟关节融合治疗Muller-Weiss病疗效分析. 南昌大学学报(医学版). 2023(03): 39-43 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
