Mechanism of macrophage extracellular traps induced activation of fibroblast-like synoviocytes in the pathogenesis of rheumatoid arthritis
-
摘要:目的
研究巨噬细胞胞外诱捕网(METs)对成纤维样滑膜细胞(FLSs)的作用和相关机制。
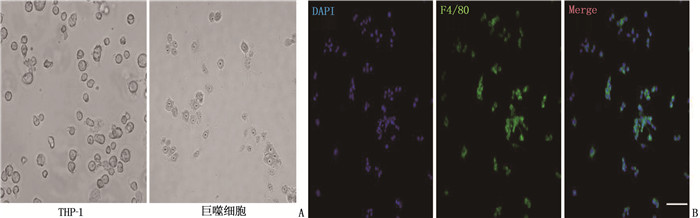
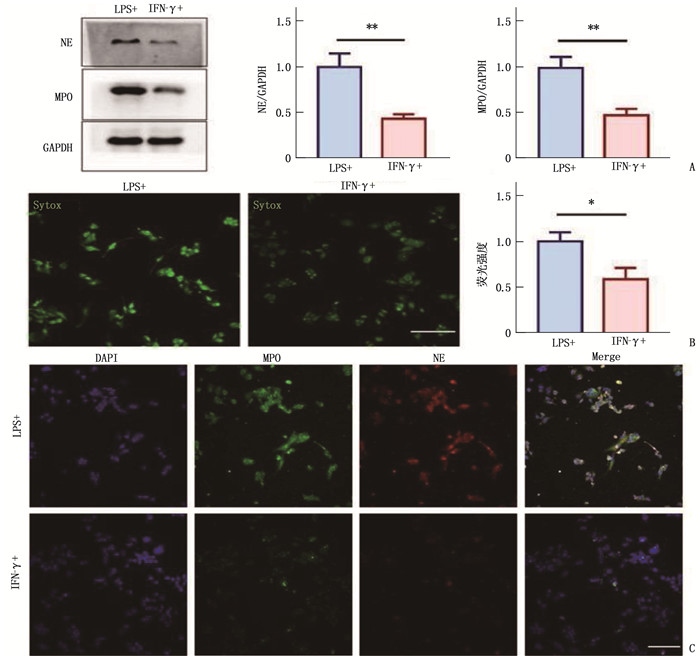
方法采用人源单核细胞白血病细胞(THP-1)系体外诱导成巨噬细胞, 分别使用脂多糖(LPS)和干扰素γ(IFN-γ)诱导METs形成。采用Sytox标记外泄的DNA水平; 采用Western blot和免疫荧光法检测METs主要蛋白成分NE和MPO。纯化METs蛋白组分,将人FLSs细胞系分为对照组、METs组、C29组、IAXO-102组和C29+IAXO-102联合干预组,除对照组外,其余组均将纯化的METs蛋白组分加入FLSs培养液中, C29组同时加入Toll样受体(TLR)2抑制剂C29, IAXO-102组加入TLR4抑制剂IAXO-102, C29+IAXO-102联合干预组同时加入C29和IAXO-102。24h后,检测FLSs细胞活力、迁移和侵袭能力。酶联免疫吸附试验(ELISA)检测培养上清中白细胞介素6(IL-6)、基质金属蛋白酶1(MMP-1)、基质金属蛋白酶3(MMP-3)和基质金属蛋白酶13(MMP-13)水平。
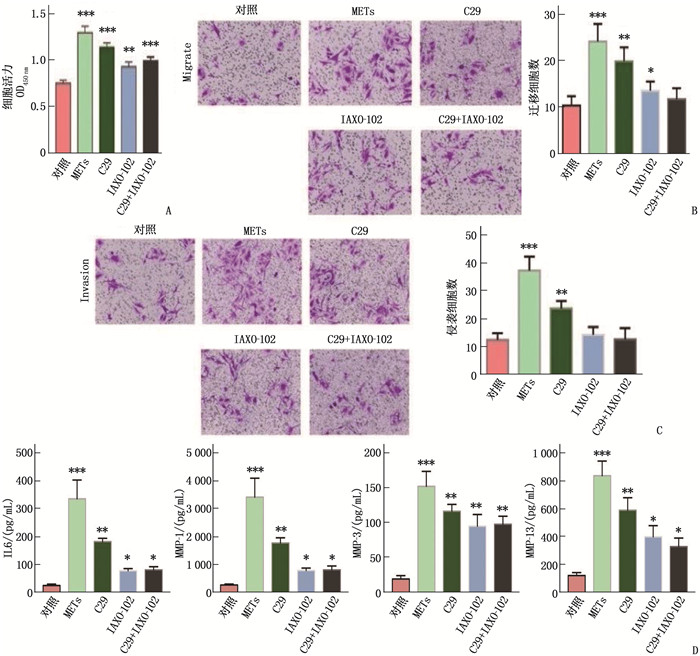
结果LPS诱导METs能力强于IFN-γ, 差异有统计学意义(P < 0.05);METs蛋白可以显著增强FLSs细胞活力、滑膜细胞迁移和侵袭能力(P < 0.05);与对照组相比, METs蛋白处理组上清中IL-6、MMP-1、MMP-3和MMP-13升高,差异有统计学意义(P < 0.05)。C29和IAXO-102单用或联用干预能显著降低滑膜细胞的活力、迁移和侵袭能力(P < 0.05);与METs组相比,C29+IAXO-102联合干预组上清中IL-6、MMP-1、MMP-3和MMP-13水平下降,差异具有统计学意义(P < 0.05), 且IAXO-102抑制能力强于C29(P < 0.05)。
结论METs蛋白作为损伤相关模式分子主要通过活化TLR4来进一步激活FLSs, 从而参与RA发病。
Abstract:ObjectiveTo investigate the role of macrophage extracellular traps (METs) on fibroblast-like synoviocytes (FLSs) and related mechanisms.
MethodsA human acute monocytic leukemia cell (THP-1) line was used to induce macrophages in vitro, and lipopolysaccharide (LPS) and interferon γ(IFN-γ) were used to induce METs; Sytox was used to mark the level of leaked DNA; Western blot and immunofluorescence were used to detect NE and MPO in METs. After purification of METs protein components, human FLSs cell lines were divided into control group, METs group, C29 group, IAXO-102 group and C29+IAXO-102 combined intervention group. Except for the control group, purified METs protein components were added to FLSs medium in the other groups. Toll-like receptor (TLR) 2 inhibitor C29 was added to the C29 group, TLR4 inhibitor IAXO-102 was added to the IAXO-102 group, and C29 and IAXO-102 were added to the C29+IAXO-102 combined intervention group. After 24 h, the viability, migration and invasion ability of FLSs cells were detected. The levels of interleukin-6 (IL-6), matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 3 (MMP-3) and matrix metalloproteinase 13 (MMP-13) in the culture supernatant were determined by enzyme-linked immunosorbent assay (ELISA).
ResultsLPS induced METs ability was significantly stronger than IFN-γ (P < 0.05). METs protein significantly enhanced FLSs cell viability, synovial cell migration and invasion ability (P < 0.05); compared with the control group, IL-6, MMP-1, MMP-3 and MMP-13 in the supernatant of METs protein treatment group were significantly increased (P < 0.05). C29 or IAXO-102 alone or their combination could significantly reduce the viability, migration and invasion ability of synovial cells (P < 0.05); compared with METs group, the levels of IL-6, MMP-1, MMP-3 and MMP-13 in the combined intervention group were significantly decreased (P < 0.05), and the inhibitory effect of IAXO-102 was significantly stronger than that of C29 (P < 0.05).
ConclusionMETs proteins as damage-related model molecules are mainly involved in RA pathogenesis by activating TLR4 to further activate FLSs.
-
-
图 3 METs通过TLR2和TLR4促进FLSs活化
A: CCK-8检测各组FLSs细胞的活力; B: 不同处理条件下的FLSs迁移能力结晶紫染色代表性图像,标尺为100 μm; C: 不同处理条件下的FLSs侵袭能力结晶紫染色代表性图像,标尺为100 μm; D: ELISA检测各组FLSs培养上清细胞产生IL-6、MMP-1、MMP-3和MMP-13的水平; 与METs单用比较, C29和IAXO-102单用或联用干预后FLSs的活力下降,细胞迁移和侵袭能力减弱,上清IL-6、MMP-1、MMP-3和MMP-13水平增高; 与C29组比较, IAXO-102组抑制能力更强, *P < 0.05, **P<0.01, ***P<0.001。
-
[1] WASSERMAN A M. Diagnosis and management of rheumatoid arthritis[J]. Am Fam Physician, 2011, 84(11): 1245-1252.
[2] SCHERER H U, HÄUPL T, BURMESTER G R. The etiology of rheumatoid arthritis[J]. J Autoimmun, 2020, 110: 102400. doi: 10.1016/j.jaut.2019.102400
[3] BERGOT A S, GIRI R, THOMAS R. The microbiome and rheumatoid arthritis[J]. Best Pract Res Clin Rheumatol, 2019, 33(6): 101497. doi: 10.1016/j.berh.2020.101497
[4] PAPAYANNOPOULOS V. Neutrophil extracellular traps in immunity and disease[J]. Nat Rev Immunol, 2018, 18(2): 134-147. doi: 10.1038/nri.2017.105
[5] DOSTER R S, ROGERS L M, GADDY J A, et al. Macrophage extracellular traps: a scoping review[J]. J Innate Immun, 2018, 10(1): 3-13. doi: 10.1159/000480373
[6] BRINKMANN V, REICHARD U, GOOSMANN C, et al. Neutrophil extracellular traps kill bacteria[J]. Science, 2004, 303(5663): 1532-1535. doi: 10.1126/science.1092385
[7] MÖLLERHERM H, VON KÖCKRITZ-BLICKWEDE M, BRANITZKI-HEINEMANN K. Antimicrobial activity of mast cells: role and relevance of extracellular DNA traps[J]. Front Immunol, 2016, 7: 265.
[8] JE S, QUAN H L, YOON Y, et al. Mycobacterium massiliense induces macrophage extracellular traps with facilitating bacterial growth[J]. PLoS One, 2016, 11(5): e0155685. doi: 10.1371/journal.pone.0155685
[9] NYGAARD G, FIRESTEIN G S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes[J]. Nat Rev Rheumatol, 2020, 16(6): 316-333. doi: 10.1038/s41584-020-0413-5
[10] MCINNES I B, SCHETT G. The pathogenesis of rheumatoid arthritis[J]. N Engl J Med, 2011, 365(23): 2205-2219. doi: 10.1056/NEJMra1004965
[11] MICHELS K R, LUKACS N W, FONSECA W. TLR activation and allergic disease: early life microbiome and treatment[J]. Curr Allergy Asthma Rep, 2018, 18(11): 61. doi: 10.1007/s11882-018-0815-5
[12] KYBURZ D, RETHAGE J, SEIBL R, et al. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling[J]. Arthritis Rheum, 2003, 48(3): 642-650. doi: 10.1002/art.10848
[13] ALSOUSI A A, IGWE O J. Redox-active trace metal-induced release of high mobility group box 1(HMGB1) and inflammatory cytokines in fibroblast-like synovial cells is Toll-like receptor 4 (TLR4) dependent[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864(11): 3847-3858. doi: 10.1016/j.bbadis.2018.08.029
[14] WU R, LONG L, CHEN Q Q, et al. Effects of Tim-3 silencing on the viability of fibroblast-like synoviocytes and lipopolysaccharide-induced inflammatory reactions[J]. Exp Ther Med, 2017, 14(3): 2721-2727. doi: 10.3892/etm.2017.4819




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号