Establishment and evaluation of Cox proportional-hazards prediction model for secondary intracranial hypertension in perioperative period in patients with acute subdural hematoma
-
摘要:目的
构建急性硬膜下血肿(ASDH)患者围术期继发颅内高压的Cox比例风险预测模型,并对其效能进行验证。
方法回顾性收集78例ASDH患者的临床资料,按围术期继发颅内高压情况将其分为继发组(25例,围术期继发颅内高压)和对照组(53例,围术期未继发颅内高压)。比较2组患者人口统计学指标、合并症、临床生化指标及影像学资料等因素的差异。应用Cox比例风险模型对ASDH患者围术期继发颅内高压的可能影响因素进行多因素分析。建立ASDH患者围术期继发颅内高压的预测模型并测算Harrell′s C指数以评估模型预测的准确度。通过列线图及校准曲线评估该模型预测风险概率与实际风险概率的符合程度。
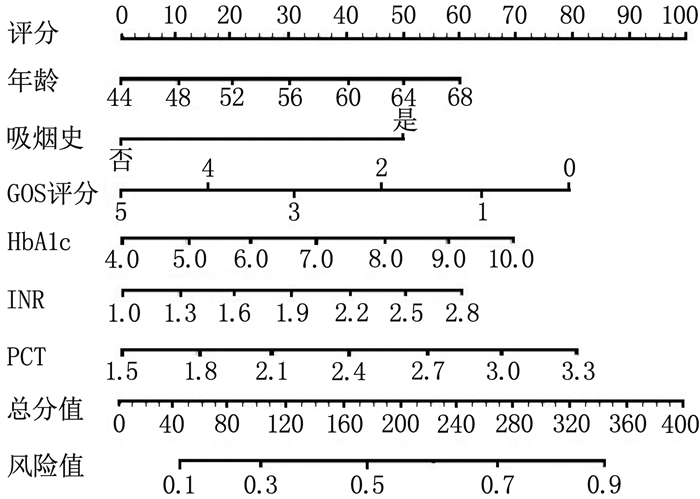
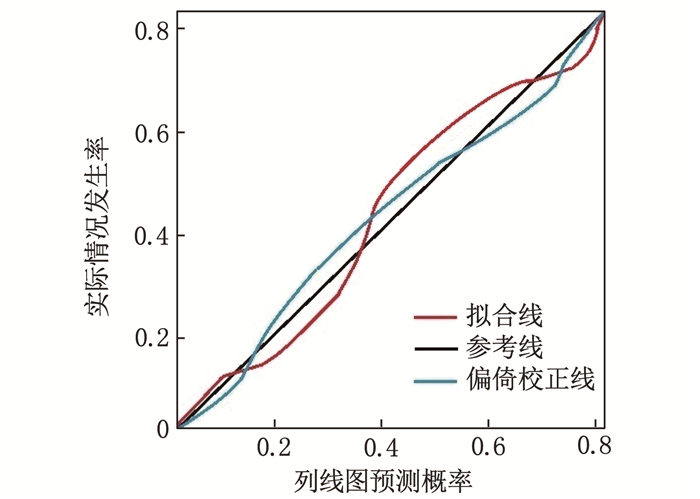
结果全组半年随访率为89.74%(70/78)。继发组年龄、吸烟史、高血压病、糖尿病、术前格拉斯哥昏迷量表(GCS) 评分、格拉斯哥预后量表(GOS) 评分、复合型血肿、颅内血肿体积、平均动脉压、糖化血红蛋白(HbA1c)、国际标准化比值(INR)、白细胞介素-6(IL-6)、降钙素原(PCT) 与对照组比较,差异有统计学意义(P < 0.05)。年龄(OR=2.895, 95%CI: 1.264~6.633, P=0.022)、吸烟史(OR=2.146, 95%CI: 1.029~4.475, P=0.036)、GOS评分(OR=0.288, 95%CI: 0.112~0.741, P=0.015)、HbA1c(OR=3.325, 95%CI: 1.243~8.894, P=0.028)、INR(OR=2.746, 95%CI: 1.203~6.267, P=0.027)、PCT(OR=3.426, 95%CI: 1.335~8.795, P=0.019)是ASDH患者围术期继发颅内高压的独立影响因素, Harrell′s C指数为0.812(95%CI: 0.789~0.872)。列线图及校准曲线显示实际风险与模型预测风险存在较好的一致性。
结论急性硬膜下血肿患者的Cox比例风险模型预测围术期继发颅内高压风险的准确度高,适宜临床推广。
Abstract:ObjectiveTo construct a Cox proportional hazards prediction model for secondary intracranial hypertension in patients with acute subdural hematoma (ASDH) during the perioperative period and validate its effectiveness.
MethodsClinical data of 78 patients with ASDH were retrospectively collected and divided into secondary group (25 cases with secondary intracranial hypertension during perioperation) and control group (53 cases without secondary intracranial hypertension during perioperation). Differences in demographic indicators, comorbidities, clinical biochemical indicators, and imaging data between the two groups were compared. The Cox proportional hazards model was used to perform a multivariate analysis of independent risk factors that may affect secondary intracranial hypertension in ASDH patients during the perioperative period. A prediction model for secondary intracranial hypertension in ASDH patients during the perioperative period was established, and Harrell′s C index was calculated to assess the predictive accuracy of the model. The degree of agreement between the model prediction and actual risks was evaluated through a nomogram and calibration curve.
ResultsThe six-month follow-up rate was 89.74% (70/78). Age, smoking history, hypertension, diabetes, preoperative Glasgow Coma Scale (GCS) score, Glasgow Outcome Scale (GOS) score, complex hematoma, intracranial hematoma volume, mean arterial pressure, glycated hemoglobin (HbA1c), international normalized ratio (INR), interleukin-6 (IL-6), and procalcitonin (PCT) in the secondary group showed statistically significant differences compared with the control group (P < 0.05). Age(OR=2.895; 95%CI, 1.264 to 6.633; P=0.022), smoking history (OR=2.146; 95%CI, 1.029 to 4.475; P=0.036), GOS score (OR=0.288; 95%CI, 0.112 to 0.741; P=0.015), HbA1c (OR=3.325; 95%CI, 1.243 to 8.894; P=0.028), INR (OR=2.746; 95%CI, 1.203 to 6.267; P=0.027), and PCT (OR=3.426; 95%CI, 1.335 to 8.795; P=0.019) were independent influencing factors for secondary intracranial hypertension in ASDH patients during the perioperative period. Harrell′s C index was 0.812 (95%CI, 0.789 to 0.872). The nomogram and calibration curve showed good consistency between the actual risk and the model prediction.
ConclusionCox proportional hazards model for patients with acute subdural hematoma has high accuracy in predicting the risk of secondary intracranial hypertension during the perioperative period and is suitable for clinical promotion.
-
-
表 1 影响围术期颅内高压发生的单因素分析(x±s)[n(%)][M(P25, P75)]
因素 继发组(n=25) 对照组(n=53) χ2/t/Z P 男 14(56.0) 37(69.8) 1.432 0.231 女 11(44.0) 16(30.2) 年龄/岁 52.8±9.5 60.4±10.3 3.402 0.003 吸烟史 15(60.0) 18(34.0) 4.718 0.030 酗酒史 9(36.0) 14(26.4) 0.751 0.386 脑血管病家族史 8(32.0) 11(20.8) 1.166 0.280 高血压病 16(64.0) 18(34.0) 6.233 0.013 糖尿病 13(52.0) 14(26.4) 4.913 0.027 术前格拉斯哥昏迷量表评分/分 8.5±2.1 11.8±2.4 5.889 < 0.001 格拉斯哥预后量表评分/分 2.5±0.4 3.8±1.1 5.717 < 0.001 复合型血肿 15(60.0) 18(34.0) 4.718 0.030 颅内血肿体积/mm3 35.5±9.6 24.8±7.5 5.364 < 0.001 术前瞳孔散大 7(28.0) 5(9.4) 0.439 0.508 平均动脉压/mmHg 185.2±20.6 172.7±18.4 2.694 0.009 糖化血红蛋白/% 7.5±2.6 5.8±1.9 3.265 0.002 红细胞计数/(×109/L) 3.5(2.7, 4.6) 3.3(2.5, 3.8) 1.389 0.327 血红蛋白/(g/L) 123.5(87.7, 144.0) 117.5(79.8, 138.2) 0.822 0.406 血小板计数/(×109/L) 83.5(56.4, 128.2) 86.2(58.6, 134.3) 0.412 0.515 白细胞计数/(×109/L) 5.5(3.7, 12.9) 6.0(4.8, 13.8) 1.756 0.243 中性粒细胞计数/(×109/L) 3.4(2.3, 6.0) 4.0(2.8, 10.2) 2.342 0.175 淋巴细胞计数/(×109/L) 1.0(0.7, 1.4) 0.8(0.4, 1.2) 0.471 0.489 凝血酶原时间/s 20.2±3.1 19.1±2.6 1.638 0.106 活化部分凝血活酶时间/s 46.2±4.3 44.5±3.7 1.797 0.076 国际标准化比值 2.1(1.5, 2.8) 1.5(1.0, 1.7) 4.289 < 0.001 D-二聚体/(μg/mL) 9.9(5.5, 10.4) 10.5(8.1, 12.4) 0.372 0.613 尿素氮/(mmol/L) 6.2±1.2 5.7±1.6 1.387 0.169 肌酐/(mmol/L) 104.3±15.8 96.8±17.4 1.828 0.071 丙氨酸氨基转移酶/(U/L) 44.5±7.6 41.4±6.8 1.809 0.074 天门冬氨酸氨基转移酶/(U/L) 48.3±6.4 45.8±7.4 1.451 0.151 总蛋白/(g/L) 58.8±4.7 61.0±5.4 1.747 0.084 白蛋白/(g/L) 23.8±2.7 25.1±2.9 1.888 0.063 总胆红素/(μmol/L) 42.8(23.7, 61.2) 50.3(26.6, 71.7) 0.953 0.368 甘油三酯/(mmol/L) 1.25±0.31 1.36±0.24 1.717 0.090 总胆固醇/(mmol/L) 2.83±0.68 2.58±0.74 1.428 0.157 低密度脂蛋白/(mmol/L) 1.75±0.41 1.57±0.39 1.871 0.065 高密度脂蛋白/(mmol/L) 0.95±0.21 1.02±0.34 1.745 0.085 血钾/(mmol/L) 3.8±0.7 4.1±0.8 1.606 0.112 血钠/(mmol/L) 138.5±3.8 140.1±3.5 1.850 0.068 白细胞介素-6/(pg/mL) 365.8(123.5, 690.4) 92.1(34.7, 232.8) 4.724 < 0.001 降钙素原/(ng/mL) 2.9±0.4 1.8±0.2 16.244 < 0.001 C反应蛋白/(mg/L) 17.8(7.5, 33.6) 16.5(6.9, 29.7) 0.480 0.482 1 mmHg=0.133 322 kPa。 表 2 Cox比例风险模型对围术期颅内高压发生的多因素分析
因素 回归系数 标准误 Wald系数 OR P 95%CI 年龄 1.063 0.423 6.315 2.895 0.022 1.264~6.633 吸烟史 0.764 0.375 4.146 2.146 0.036 1.029~4.475 术前格拉斯哥昏迷量表评分 0.772 0.468 2.724 2.165 0.069 0.865~5.418 格拉斯哥预后量表评分 -1.244 0.482 6.670 0.288 0.015 0.112~0.741 复合型血肿 0.766 0.556 1.900 2.152 0.105 0.723~6.399 颅内血肿体积 0.686 0.436 2.476 1.986 0.091 0.845~4.663 平均动脉压 0.710 0.521 1.860 2.035 0.101 0.733~5.650 糖化血红蛋白 1.201 0.502 5.728 3.325 0.028 1.243~8.894 国际标准化比值 1.010 0.421 5.757 2.746 0.027 1.203~6.267 白细胞介素-6 0.966 0.540 3.202 2.628 0.061 0.911~7.573 降钙素原 1.230 0.481 6.554 3.426 0.019 1.335~8.795 -
[1] 胡晓芳, 赵琳, 张尚明, 等. 颅内压监测下降阶梯减压技术在创伤后脑疝患者术中的应用[J]. 中华神经创伤外科电子杂志, 2020, 6(4): 254-256. [2] SHIN D S, HWANG S C. Neurocritical management of traumatic acute subdural hematomas[J]. Korean J Neurotrauma, 2020, 16(2): 113-125.
[3] HADDADIN F, MUNOZ ESTRELLA A, HERZOG E. Hypertensive emergency presenting with acute spontaneous subdural hematoma[J]. J Cardiol Cases, 2019, 19(1): 25-28.
[4] JUSKYS R, VILCINIS R, PILIPONIS L, et al. Degree of basal cisterns compression predicting mortality and functional outcome after craniotomy and primary decompressive craniectomy in acute subdural hematoma population[J]. Acta Neurochir, 2023, 165(12): 4013-4020.
[5] WEN P, XU W L, CHEN H. Intracranial hemorrhage following drainage of chronic subdural effusion and hematoma: a case report and review of the literature[J]. Ibrain, 2022, 8(1): 68-77.
[6] PASTOR I S, DUMBRAVǍ L P, SISERMAN C, et al. Predictive factors of 30-day mortality in patients with traumatic subdural hematoma[J]. Exp Ther Med, 2021, 22(1): 757.
[7] TSUCHIYA R, OOIGAWA H, KIMURA T, et al. Study of certain easily available biochemical markers in prognostication in severe traumatic brain injury requiring surgery[J]. Surg Neurol Int, 2023, 14: 410.
[8] O′NEILL B E, WOZNY T, ULUC K, et al. Acute nontraumatic subdural hematoma from ruptured accessory meningeal artery pseudoaneurysm[J]. Surg Neurol Int, 2021, 12: 186.
[9] GODDARD C, COLLOPY K T, POWERS IV W F. Prehospital hypertonic saline administration after severe traumatic brain injury[J]. Air Med J, 2022, 41(5): 498-502.
[10] BEUCLER N, SELLIER A, JOUBERT C, et al. Severe trauma patients requiring undelayable combined cranial and extra-cranial surgery: a proof-of-concept monocentric study[J]. Mil Med, 2022, 187(9/10): 1127-1135.
[11] KIEU H D, LE T D, HOANG T M. Acute spontaneous subdural hematoma secondary to ruptured arteriovenous malformation: a rare entity[J]. Ann Med Surg, 2021, 68: 102613.
[12] FOOTE C W, JARVIS S, DOAN X L, et al. Correlation between intracranial pressure monitoring for severe traumatic brain injury with hospital length of stay and discharge disposition: a retrospective observational cohort study[J]. Patient Saf Surg, 2022, 16(1): 40.
[13] OU J B, YAO L Q, FU Y T, et al. Nomograms for the prediction of decannulation in patients with neurological injury: a study based on clinical practice[J]. Int J Neurosci, 2023: 1-9.
[14] AND APPLICATIONS B C. Retracted: Diagnostic Accuracy of Procalcitonin for Bacterial Infection in Liver Failure: A Meta-Analysis [J]. Bioinorg Chem Appl, 2023, 2023: 9824505.
[15] JIMOH A K, BOLAJI O B, ADELEKAN A, et al. Clinical utility of procalcitonin and C-reactive protein in the management of neonatal sepsis in a resource-limited Nigerian hospital[J]. Niger J Clin Pract, 2023, 26(12): 1895-1901.
[16] CARABIAS C S, GOMEZ P A, PANERO I, et al. Chitinase-3-like protein 1, serum amyloid A1, C-reactive protein, and procalcitonin are promising biomarkers for intracranial severity assessment of traumatic brain injury: relationship with Glasgow Coma scale and computed tomography volumetry[J]. World Neurosurg, 2020, 134: e120-e143.
[17] WASFIE T, YOUNG C, NAISAN M, et al. Acute traumatic subdural hematoma in the elderly and associated factors that may influence chronicity[J]. Am Surg, 2023, 89(12): 6312-6314.
[18] WASFIE T, FITZPATRICK N, NIASAN M, et al. Factors favoring the development of chronic subdural hematoma after traumatic acute subdural hematoma in the elderly[J]. Am Surg, 2022, 88(3): 372-375.
[19] KWINTA B, KRZYZEWSKI R M, KLIS K, et al. Prolonged international normalized ratio is independently associated with recurrence of acute subdural hematoma needing reoperation[J]. Folia Med Cracov, 2018, 58(1): 53-56.
[20] PASTOR I S, PARA I, VESA Ş C, et al. The impact of oral anticoagulants on the characteristics of subdural hematomas and other brain lesions in patients with traumatic brain injury[J]. Med Pharm Rep, 2023, 96(3): 269-273.
[21] 李峥, 唐晓平, 张亚兰, 等. 预减压联合标准去大骨瓣减压对创伤性急性硬膜下血肿合并脑疝的疗效分析[J]. 中国急救复苏与灾害医学杂志, 2020, 15(11): 1266-1269. -
期刊类型引用(11)
1. 连利媛,张玉. 新生儿呼吸窘迫综合征患儿预后情况及不良预后影响因素分析. 航空航天医学杂志. 2024(09): 1124-1126 .  百度学术
百度学术
2. 邹晴,方玉玲,彭晓瑞,谢双霞,胡艳松. 新生儿呼吸窘迫综合征患儿预后不良的影响因素. 中国民康医学. 2024(21): 8-11 .  百度学术
百度学术
3. 季俊玲,木菁菁,温苗苗,赵碧茹. 新生儿呼吸窘迫综合征患儿肺出血发生情况及预后的影响因素分析. 中国妇幼保健. 2023(18): 3518-3522 .  百度学术
百度学术
4. 杜睿,甄丽. 肺部超声评估在新生儿呼吸窘迫综合征中的临床应用价值. 国际呼吸杂志. 2023(09): 1077-1082 .  百度学术
百度学术
5. 徐刘毅. 早期应用肺表面活性物质预防新生儿呼吸窘迫综合征的效果分析. 中国妇幼保健. 2023(24): 4858-4861 .  百度学术
百度学术
6. 李卓娅,宋红,宋焕清,周川,李晶晶. 氨基末端脑钠肽前体水平在早产儿支气管肺发育不良中的临床价值. 实用临床医药杂志. 2022(10): 83-87+96 .  本站查看
本站查看
7. 李紫薇,顾美群,许小志,唐莲芳,许小艳,杨景晖,毕凯,米弘瑛. 某三甲医院101例极早产儿临床资料分析. 昆明医科大学学报. 2022(06): 85-91 .  百度学术
百度学术
8. 刘立静,马洪欣,杜睿,邸晓玲,顾华. 肺部超声新评分法在新生儿呼吸窘迫综合征病情评价及治疗中的应用效果. 实用临床医药杂志. 2022(21): 111-114 .  本站查看
本站查看
9. 罗恒,梅玥婧,刘夕珑,张立英,卢丹. 保留胎膜囊剖宫产术在早产双胎妊娠中的应用评价. 实用临床医药杂志. 2022(23): 60-64 .  本站查看
本站查看
10. 李婕. 不同剂量牛肺表面活性剂治疗新生儿呼吸窘迫综合征30例疗效观察. 药品评价. 2021(15): 939-941 .  百度学术
百度学术
11. 李梦娇,李晶,马金红,张迪. 个性化的互动延续性护理干预对低出生体重早产儿体格生长的影响研究. 中国优生与遗传杂志. 2021(12): 1782-1785 .  百度学术
百度学术
其他类型引用(2)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号