Mechanism of liver-soothing and stomach-harmonizing recipe alleviating airway inflammation in rats with gastroesophageal reflux disease by regulating PPAR-γ/RXR signaling pathway
-
摘要:目的
探讨疏肝和胃方通过调控过氧化物酶体增殖物激活受体γ/类视黄醇X受体(PPAR-γ/RXR)信号通路缓解胃食管反流病(GERD)大鼠气道炎症的机制。
方法采用食管下端盐酸灌注法构建GERD合并气道炎症大鼠模型。将50只SD雄性大鼠随机分为对照组、模型组、疏肝和胃方低剂量组(10.49 g/kg生药量)、疏肝和胃方高剂量组(20.98 g/kg生药量)和奥美拉唑组(3.67 mg/kg), 每组10只, 灌胃14 d。采用苏木精-伊红(HE)染色观察气管组织的病理学变化; 采用RT-qPCR检测支气管肺泡灌洗液中炎症因子[白细胞介素(IL)-17、IL-33、诱导型一氧化氮合酶(iNOS)]以及抗炎因子[IL-10、克拉拉细胞蛋白16(CC16)、表面活性蛋白-D(SP-D)]的mRNA表达水平; 采用免疫印迹法检测PPAR-γ、RXR-α、核因子-κB(NF-κB)、活化蛋白-1(AP-1)的蛋白相对表达量。
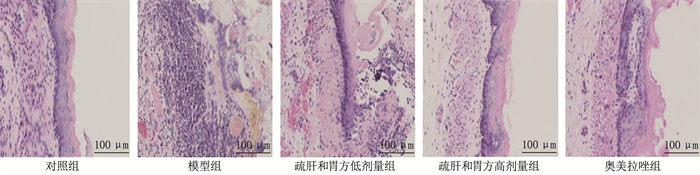
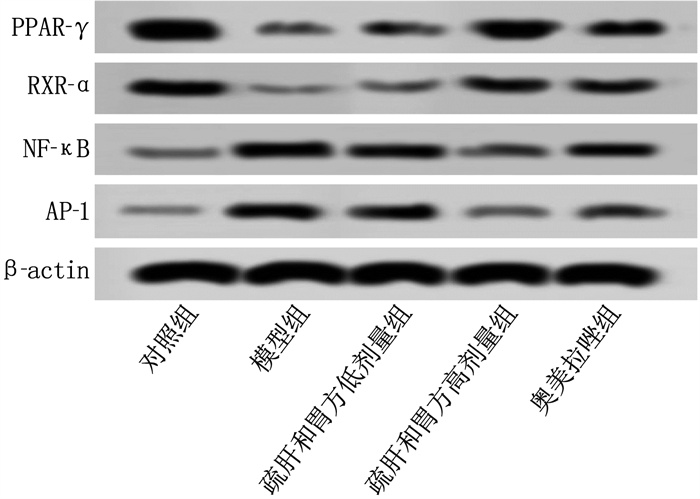
结果HE染色结果显示,模型组可见大量炎症细胞浸润,疏肝和胃方低剂量组、高剂量组和奥美拉唑组炎症细胞浸润明显减少。与对照组比较,模型组支气管肺泡灌洗液中IL-17、IL-33、iNOS的mRNA表达水平升高, IL-10、CC16、SP-D的mRNA表达水平降低, PPAR-γ、RXR-α的蛋白相对表达量升高, NF-κB、AP-1的蛋白相对表达量降低,差异均有统计学意义(P < 0.05)。与模型组比较,疏肝和胃方高剂量组和奥美拉唑组上述指标均有改善,差异有统计学意义(P < 0.05)。
结论疏肝和胃方可以有效缓解GERD大鼠气道炎症,其作用机制可能与激活PPARγ/RXR信号通路有关。
-
关键词:
- 疏肝和胃方 /
- 胃食管反流病 /
- 过氧化物酶体增殖物激活受体 /
- 类视黄醇X受体 /
- 信号通路
Abstract:ObjectiveTo investigate the mechanism of liver-soothing and stomach-harmonizing recipe alleviating airway inflammation in rats with gastroesophageal reflux disease (GERD) by regulating peroxisome proliferator-activated receptor γ/retinoid X receptor (PPAR-γ/RXR) signaling pathway.
MethodsA rat model of GERD with airway inflammation was established by perfusion of hydrochloric acid into the lower esophagus. A total of 50 SD male rats were randomly divided into control group, model group, low-dose liver-soothing and stomach-harmonizing recipe group (10.49 g/kg crude drug dosage), high-dose liver-soothing and stomach-harmonizing recipe group (20.98 g/kg crude drug dosage), and omeprazole group (3.67 mg/kg), with 10 rats in each group. The rats were gavaged for 14 days. Hematoxylin and eosin (HE) staining was used to observe the pathological change in tracheal tissue. RT-qPCR was used to detect the mRNA expression levels of inflammatory cytokines[interleukin (IL)-17, IL-33, inducible nitric oxide synthase (iNOS)]and anti-inflammatory cytokines[IL-10, Clara cell 16-kDa protein (CC16), surfactant protein-D (SP-D)]in bronchoalveolar lavage fluid; the Western blot was used to detect the relative protein expression levels of PPAR-γ, RXR-α, nuclear factor-κB (NF-κB), and activator protein-1 (AP-1).
ResultsThe HE staining results showed that a large number of inflammatory cell infiltrations were observed in the model group, while the inflammatory cell infiltrations were significantly reduced in the low-dose liver-soothing and stomach-harmonizing recipe group, high-dose liver-soothing and stomach-harmonizing recipe group, and omeprazole group. Compared with the control group, the mRNA expression levels of IL-17, IL-33 and iNOS in bronchoalveolar lavage fluid were significantly increased while the mRNA expression levels of IL-10, CC16 and SP-D were significantly decreased in the model group, and the relative protein expression levels of PPAR-γ and RXR-α were significantly increased, while the relative protein expression levels of NF-κB and AP-1 were significantly decreased in the model group (P < 0.05). Compared with the model group, the above indicators were significantly improved in the high-dose liver-soothing and stomach-harmonizing recipe group and omeprazole group (P < 0.05).
ConclusionLiver-soothing and stomach-harmonizing recipe can effectively alleviate airway inflammation in rats with GERD, and its mechanism may be related to the activation of the PPAR-γ/RXR signaling pathway.
-
-
表 1 各组大鼠支气管肺泡灌洗液中促炎症介质表达水平比较(x±s)
组别 n IL-17 mRNA IL-33 mRNA iNOS mRNA 对照组 10 0.86±0.03 0.63±0.10 0.98±0.12 模型组 10 2.04±0.32* 0.91±0.05* 2.21±0.30* 疏肝和胃方低剂量组 10 1.88±0.14 0.82±0.13 1.78±0.24# 疏肝和胃方高剂量组 10 0.87±0.09# 0.61±0.02# 1.42±0.11# 奥美拉唑组 10 0.94±0.07# 0.65±0.10# 1.57±0.10# IL-17 : 白细胞介素-17; IL-33 : 白细胞介素-33; iNOS: 诱导型一氧化氮合酶。与对照组比较, * P < 0.05; 与模型组比较, #P < 0.05。 表 2 各组大鼠支气管肺泡灌洗液中抗炎症介质表达水平比较(x±s)
组别 n IL-10 mRNA CC16 mRNA SP-D mRNA 对照组 10 0.94±0.19 1.07±0.16 0.96±0.08 模型组 10 0.38±0.04* 0.44±0.06* 0.43±0.07* 疏肝和胃方低剂量组 10 0.60±0.10# 0.53±0.07 0.46±0.05 疏肝和胃方高剂量组 10 0.79±0.20# 0.87±0.13# 0.82±0.05# 奥美拉唑组 10 0.71±0.23# 0.84±0.10# 0.75±0.09# IL-10 : 白细胞介素-10; CC16 : 克拉拉细胞蛋白16; SP-D: 表面活性蛋白-D。与对照组比较, * P < 0.05; 与模型组比较, #P < 0.05。 表 3 各组大鼠支气管肺泡灌洗液中PPAR-γ、RXR-α、NF-κB、AP-1蛋白相对表达量比较(x±s)
组别 n PPAR-γ RXR-α NF-κB AP-1 对照组 10 0.20±0.02 0.57±0.06 0.38±0.02 0.22±0.03 模型组 10 0.05±0.01* 0.14±0.02* 0.89±0.13* 1.02±0.25* 疏肝和胃方低剂量组 10 0.13±0.01# 0.35±0.07# 0.65±0.06# 0.71±0.12# 疏肝和胃方高剂量组 10 0.20±0.05# 0.60±0.07# 0.53±0.09# 0.43±0.08# 奥美拉唑组 10 0.19±0.06# 0.56±0.10# 0.58±0.05# 0.33±0.06# PPAR-γ: 过氧化物酶体增殖物激活受体γ; RXR-α: 类视黄醇X受体α; NF-κB: 核因子-κB; AP-1: 活化蛋白-1。与对照组比较, * P < 0.05; 与模型组比较, #P < 0.05。 -
[1] MARET-OUDA J, MARKAR S R, LAGERGREN J. Gastroesophageal reflux disease: a review[J]. JAMA, 2020, 324(24): 2536-2547. doi: 10.1001/jama.2020.21360
[2] JOHNSTON N, ONDREY F, ROSEN R, et al. Airway reflux[J]. Ann N Y Acad Sci, 2016, 1381(1): 5-13. doi: 10.1111/nyas.13080
[3] DUNBAR K B, AGOSTON A T, ODZE R D, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes[J]. JAMA, 2016, 315(19): 2104-2112. doi: 10.1001/jama.2016.5657
[4] NGUYEN P A, ISLAM M, GALVIN C J, et al. Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia[J]. Int J Qual Health Care, 2020, 32(5): 292-299. doi: 10.1093/intqhc/mzaa041
[5] EUSEBI L H, RABITTI S, ARTESIANI M L, et al. Proton pump inhibitors: risks of long-term use[J]. J Gastroenterol Hepatol, 2017, 32(7): 1295-1302. doi: 10.1111/jgh.13737
[6] JACKSON M A, GOODRICH J K, MAXAN M E, et al. Proton pump inhibitors alter the composition of the gut microbiota[J]. Gut, 2016, 65(5): 749-756. doi: 10.1136/gutjnl-2015-310861
[7] 孙永顺, 朱生樑, 王宏伟, 等. 疏肝和胃方治疗难治性胃食管反流病的临床观察[J]. 时珍国医国药, 2016, 27(10): 2457-2459. [8] STARK J M, COQUET J M, TIBBITT C A. The role of PPAR-γ in allergic disease[J]. Curr Allergy Asthma Rep, 2021, 21(11): 45. doi: 10.1007/s11882-021-01022-x
[9] 刘春丽, 赖克方, 陈如冲, 等. 盐酸灌注豚鼠食管反流性疾病模型的建立[J]. 中国病理生理杂志, 2006, 22(3): 620-621, 624. doi: 10.3321/j.issn:1000-4718.2006.03.049 [10] FASS R, BOECKXSTAENS G E, EL-SERAG H, et al. Gastro-oesophageal reflux disease[J]. Nat Rev Dis Primers, 2021, 7(1): 55. doi: 10.1038/s41572-021-00287-w
[11] EL-SERAG H B, SWEET S, WINCHESTER C C, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review[J]. Gut, 2014, 63(6): 871-880. doi: 10.1136/gutjnl-2012-304269
[12] MEHTA R S, NGUYEN L H, MA W J, et al. Association of diet and lifestyle with the risk of gastroesophageal reflux disease symptoms in US women[J]. JAMA Intern Med, 2021, 181(4): 552-554. doi: 10.1001/jamainternmed.2020.7238
[13] LI Z, TAO L, ZHANG S S, et al. Modified Xiaochaihu Decoction for gastroesophageal reflux disease: a randomized double-simulation controlled trial[J]. World J Gastroenterol, 2021, 27(28): 4710-4721. doi: 10.3748/wjg.v27.i28.4710
[14] SONG S Z, ZHANG Y S, ZHANG J W, et al. Efficacy evaluation and exploratory analysis of influencing factors of Banxia Houpu Decoction in the treatment of refractory gastroesophageal reflux disease[J]. Medicine, 2024, 103(24): e38045. doi: 10.1097/MD.0000000000038045
[15] CHENG Y, KOU F S, ZHANG X S, et al. Network pharmacology analysis of hewei jiangni granule for gastroesophageal reflux disease and experimental verification of its anti-neurogenic inflammation mechanism[J]. Drug Des Devel Ther, 2022, 16: 1349-1363. doi: 10.2147/DDDT.S348985
[16] 杜梦蝶, 王玮, 付文尚, 等. 疏肝和胃方通过抑制NF-κB通路调控M1巨噬细胞极化对反流性食管炎大鼠食管黏膜炎症的影响[J]. 上海中医药杂志, 2024, 58(5): 66-72. [17] 张晓轩, 翟超, 李光璨, 等. 子宫内膜容受性与白血病抑制因子的相关性[J]. 国际生殖健康计划生育杂志, 2022, 41(4): 327-331. [18] KURAMOTO K, MORISHIMA Y, YOSHIDA K, et al. Nrf2 deficiency accelerates IL-17-dependent neutrophilic airway inflammation in asthmatic mice[J]. Antioxidants, 2024, 13(7): 818. doi: 10.3390/antiox13070818
[19] MIYAOKA C, WATANABE M, NAKAMOTO K, et al. Association of IL-33 in modeling type-2 airway inflammation and pulmonary emphysema in mice[J]. Immun Inflamm Dis, 2024, 12(4): e1252. doi: 10.1002/iid3.1252
[20] HAN X, CHAI R N, QI F F, et al. Natural helper cells mediate respiratory syncytial virus-induced airway inflammation by producing type 2 cytokines in an IL-33-dependent manner[J]. Immunotherapy, 2017, 9(9): 715-722. doi: 10.2217/imt-2017-0037
[21] NADEEM A, SIDDIQUI N, ALHARBI N O, et al. Acute glutathione depletion leads to enhancement of airway reactivity and inflammation via p38MAPK-iNOS pathway in allergic mice[J]. Int Immunopharmacol, 2014, 22(1): 222-229. doi: 10.1016/j.intimp.2014.06.030
[22] WILSON M S, ELNEKAVE E, MENTINK-KANE M M, et al. IL-13Ralpha2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice[J]. J Clin Invest, 2007, 117(10): 2941-2951. doi: 10.1172/JCI31546
[23] LIU M X, LU J J, ZHANG Q, et al. Clara cell 16 KDa protein mitigates house dust mite-induced airway inflammation and damage via regulating airway epithelial cell apoptosis in a manner dependent on HMGB1-mediated signaling inhibition[J]. Mol Med, 2021, 27(1): 11.
[24] WANG J Y, REID K B M. The immunoregulatory roles of lung surfactant collectins SP-A, and SP-D, in allergen-induced airway inflammation[J]. Immunobiology, 2007, 212(4/5): 417-425.
[25] XU J, ZHU Y T, WANG G Z, et al. The PPARγ agonist, rosiglitazone, attenuates airway inflammation and remodeling via heme oxygenase-1 in murine model of asthma[J]. Acta Pharmacol Sin, 2015, 36(2): 171-178. doi: 10.1038/aps.2014.128
[26] UCHIMURA K, NAKAMUTA M, ENJOJI M, et al. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells[J]. Hepatology, 2001, 33(1): 91-99. doi: 10.1053/jhep.2001.21145
[27] HAYDEN M S, GHOSH S. Shared principles in NF-kappaB signaling[J]. Cell, 2008, 132(3): 344-362. doi: 10.1016/j.cell.2008.01.020
[28] KUSIAK A, BRADY G. Bifurcation of signalling in human innate immune pathways to NF-κB and IRF family activation[J]. Biochem Pharmacol, 2022, 205: 115246. doi: 10.1016/j.bcp.2022.115246
[29] MIRZA A Z, ALTHAGAFI I I, SHAMSHAD H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications[J]. Eur J Med Chem, 2019, 166: 502-513. doi: 10.1016/j.ejmech.2019.01.067
[30] DE SOUZA BASSO B, HAUTE G V, ORTEGA-RIBERA M, et al. Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-γ and NF-κB mechanism[J]. J Ethnopharmacol, 2021, 280: 114433. doi: 10.1016/j.jep.2021.114433
[31] WANG N P, VERNA L, CHEN N G, et al. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells[J]. J Biol Chem, 2002, 277(37): 34176-34181. doi: 10.1074/jbc.M203436200
[32] YAN L, ZHANG J D, WANG B, et al. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin Ⅱ-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity[J]. PLoS One, 2013, 8(9): e72548. doi: 10.1371/journal.pone.0072548
[33] MENG X, SUN X R, ZHANG Y H, et al. PPARγ agonist PGZ attenuates OVA-induced airway inflammation and airway remodeling via RGS4 signaling in mouse model[J]. Inflammation, 2018, 41(6): 2079-2089. doi: 10.1007/s10753-018-0851-2
[34] TRIFILIEFF A, BENCH A, HANLEY M, et al. PPAR-α and-γ but not-δ agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-κB-independent effect[J]. British J Pharmacology, 2003, 139(1): 163-171. doi: 10.1038/sj.bjp.0705232
[35] KOBAYASHI M, THOMASSEN M J, RAMBASEK T, et al. An inverse relationship between peroxisome proliferator-activated receptor gamma and allergic airway inflammation in an allergen challenge model[J]. Ann Allergy Asthma Immunol, 2005, 95(5): 468-473. doi: 10.1016/S1081-1206(10)61173-8
-
期刊类型引用(1)
1. 彭卫卫,钟丽婷,李泽蕾,钟炳娣,聂启鸿. 亮菌口服溶液对肝癌术后患者免疫功能、肝功能及炎性因子的影响. 临床误诊误治. 2023(09): 84-88 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
