Advances for ferroptosis in treating myocardial ischemia reperfusion injury
-
摘要:
铁死亡是一种铁依赖性的脂质过氧化介导细胞膜损伤致细胞死亡的新方式, 其受多种细胞代谢途径的调控, 包括铁代谢、脂质代谢、氧化还原系统等, 许多器官的损伤和退行性病变均与其相关, 在治疗缺血性疾病和脂质过氧化相关的退行性变疾病中有巨大潜力。心肌缺血再灌注损伤(MIRI)是急性心肌梗死患者进行血运重建治疗后最常见的死亡原因, 近年的研究表明铁死亡与MIRI密切相关, 通过氧化应激、铁代谢、脂质代谢、内质网应激、炎症反应等影响MIRI, 干预再灌注过程中的铁死亡可以有效改善心功能, 减少梗死面积。本文就铁死亡在MIRI中的具体作用及相关研究进展予以综述。
Abstract:Ferroptosis, a new form of programmed cell death marked by iron-dependent phospholipid peroxidation, is regulated by complex cellular metabolic pathways, including iron metabolism, lipid metabolism, and oxidation-reduction system, is associated with many organ injuries and degeneration, and has great potential in the treatment of ischemic diseases and lipid peroxide-related degenerative diseases. Myocardial ischemia reperfusion injury (MIRI) is the most common cause of death in patients with acute myocardial infarction after revascularization therapy. Recent studies have shown that ferroptosis is intimately related to the pathological process of MIRI. Ferroptosis is associated with MIRI through oxidative stress, iron metabolism, lipid metabolism, endoplasmic reticulum stress and inflammatory response. Intervention of ferroptosis during reperfusion can effectively improve cardiac function and reduce myocardial infarct size. In this paper, the research progress was explored between ferroptosis and MIRI, and the specific role of ferroptosis in MIRI was discussed.
-
颅脑外伤是指于头颅部发生的常见外部损伤,多由跌坠伤和撞伤导致。颅内高压是导致脑外伤患者发生不良预后的主要原因,有效降压是临床抢救重点[1]。去骨瓣减压术是临床常用治疗脑外伤手术方案,能有效降低颅内压,保护脑组织,但术后颅骨缺损影响大脑神经功能与脑皮质血液灌注,对术后患者行颅骨成形术能有效改善术后不良影响[2]。硬膜下积液是脑外伤行去骨瓣减压术的常见并发症,治疗难度较高,易加重病情,增高不良预后风险[3]。本研究观察脑外伤去骨瓣减压术联合早期颅骨成形术的临床效果,并分析术后硬膜下积液的危险因素,现将结果报告如下。
1. 资料与方法
1.1 一般资料
选取2019年7月—2020年7月脑外伤行去骨瓣减压术的120例患者为研究对象,根据术后行颅骨成形术的时间分为对照组(常规时间行颅骨成形术, n=80)与研究组(早期行颅骨成形术, n=40)。对照组男50例,女30例; 年龄20~55岁,平均(43.42±7.32)岁; 体质量指数(BMI)19~28 kg/m2, 平均(22.51±2.23) kg/m2; 发病至就诊时间3~21 h, 平均(11.43±6.13) h; 致病原因为高处跌伤21例、交通事故32例、打架斗殴27例。研究组男21例,女19例; 年龄19~54岁,平均(42.57±7.12)岁; BMI为18~27 kg/m2, 平均(22.31±2.35) kg/m2; 发病至就诊时间2~20 h, 平均(12.05±5.98) h; 致病原因为高处跌伤11例、交通事故24例、打架斗殴5例。2组患者一般资料比较,差异无统计学意义(P>0.05), 具有可比性。
纳入标准: ①经诊断确诊,并行去骨瓣减压术治疗的的脑外伤患者; ②患者可正常沟通,听力与理解能力正常; ③入院时格拉斯哥昏迷评分不超过8分者; ④临床资料完整者。排除标准: ①合并严重心、肝、肺、肾疾病患者; ②既往有颅脑手术史患者; ③术后发生感染、颅位病变患者; ④术后随访失联患者。
1.2 方法
颅骨成形术使用三维朔性钛网经颅骨三维重建加工为修补材料,经高压灭菌后备用。根据患者手术部位选择合适体位,经全身麻醉后沿去骨瓣减压术切口做马蹄形切开暴露骨缘,分离骨膜和皮瓣粘连部分,修整骨缘成斜坡状,置入修补材料并固定,使用自体捏肌筋膜进行硬脑膜颞成形。冲洗创面,留置引流管,逐层缝合头皮,使用弹力绷带加压包扎。对照组患者去骨瓣减压术后3~6个月行颅骨成形术,研究组术后5~8周行颅骨成形术。2组均于术后15 d、2个月对神经、运动、日常生活功能进行评分。
1.3 观察指标
采用神经功能缺损(NIHSS)评分评价神经功能[4], 包括11个项目,共计42分,分数越高,表示神经损伤越严重。运动功能[5]采用肢体运动功能量表(Fugl-Meyer)进行评定,总分为100分, 100分为正常, 95~ < 100分为轻度障碍, 85~ < 95分为中度障碍, 50~ < 85分为明显障碍, 50分以下为重度障碍。日常生活功能[6]采用Barthel指数评分进行评定,共包括10个项目,共计100分, 70分以上为轻度障碍, >46~70分为中度障碍, >25~46分为重度障碍, ≤25分为极度障碍。记录2组患者的临床特征,包括年龄、性别、颅内血肿情况、中线移位、术前是否有脑疝、骨瓣情况等。硬膜下积液标准: CT诊断表现为积液量>5 mm, 中线移位以移位>5 mm为有移位[7]。
1.4 统计学处理
所有数据均采用统计软件SPSS 22.0进行处理。计数资料采用[n(%)]表示,行χ2检验; 计量资料采用(x±s)表示,行t检验或重复测量分析,两两比较采用LSD-t检验。组间比较行单因素方差分析,多因素采用二元Logistic回归分析。P < 0.05为差异有统计学意义。
2. 结果
2.1 2组手术前后神经功能、肢体运动能力、生活能力比较
术前, 2组NIHSS评分、Fugl-Meyer评分、Barthel评分比较,差异无统计学意义(P>0.05); 术后2个月,研究组NIHSS评分低于对照组, Fugl-Meyer评分、Barthel评分高于对照组,差异有统计学意义(P < 0.05), 见表 1。
表 1 2组手术前后神经功能、肢体运动能力、生活能力评分比较(x±s)分 指标 组别 术前 术后15 d 术后2个月 NIHSS评分 研究组(n=40) 31.22±4.11 20.84±4.95* 15.63±3.69*#△ 对照组(n=80) 31.61±3.94 23.06±4.80* 19.62±5.06*# Fugl-Meyer评分 研究组(n=40) 51.34±16.58 69.76±17.81* 82.55±19.41*#△ 对照组(n=80) 52.48±16.25 66.42±19.46* 70.20±20.81*# Barthel评分 研究组(n=40) 30.54±7.22 50.45±15.73* 76.57±22.96*#△ 对照组(n=80) 31.22±8.33 46.67±14.80* 62.53±20.87*# 与术前比较, *P < 0.05; 与术后15 d比较, #P < 0.05; 与对照组比较, △P < 0.05。 2.2 2组术后并发症发生情况比较
2组术后脑积水、癫痫、侧脑室扩大移位发生率比较,差异无统计学意义(P>0.05); 研究组硬膜下积液、颅脑缺损综合征发生率低于对照组,差异有统计学意义(P < 0.05), 见表 2。
表 2 2组术后并发症发生情况比较[n(%)]组别 脑积水 癫痫 硬膜下积液 颅脑缺损综合征 侧脑室扩大移位 研究组(n=40) 2(5.00) 4(10.00) 4(10.00)* 6(15.00)* 1(2.50) 对照组(n=80) 12(15.00) 5(6.25) 24(30.00) 26(32.50) 5(6.25) 与对照组比较, *P < 0.05。 2.3 术后硬膜下积液影响因素分析
根据患者是否发生硬膜下积液将其分为硬膜下积液组和非硬膜下积液组。2组患者年龄、性别、脑室内出血、脑池受压、硬膜下血肿、硬膜外血肿、脑疝、哥斯拉昏迷评分、去骨瓣侧边、骨瓣最长径、骨瓣最高径比较,差异无统计学意义(P>0.05); 2组患者中线移位、脑内血肿、蛛网膜撕裂、皮层切开、骨瓣边缘距中线距离、骨窗面积比较,差异有统计学意义(P < 0.05), 见表 3。
表 3 术后硬膜下积液单因素分析(x±s)[n(%)]指标 硬膜下积液组
(n=28)非硬膜下积液组
(n=92)年龄/岁 44.83±7.12 43.33±7.22 性别 男 20(71.43) 69(75.00) 女 8(28.57) 23(25.00) 脑室内出血 2(7.14) 5(5.43) 中线移位 20(71.43)* 30(32.61) 脑池受压 23(82.14) 71(77.17) 硬膜下血肿 25(89.29) 69(75.00) 硬膜外血肿 7(25.00) 33(35.87) 脑内血肿 25(89.29)* 31(33.70) 蛛网膜撕裂 25(89.29)* 56(60.87) 皮层切开 18(64.29)* 33(35.87) 脑疝 15(53.57) 33(35.87) 哥斯拉昏迷评分 3~ < 5分 15(53.57) 62(67.39) 5~ < 8分 13(46.43) 30(32.61) 去骨瓣侧边 单侧 16(57.14) 36(39.13) 双侧 12(42.86) 56(60.87) 骨瓣最长径/cm 11.19±2.14 10.64±2.38 骨瓣最高径/cm 8.17±1.53 7.84±1.46 骨瓣边缘距中线距离 ≤2 cm 19(67.86)* 31(33.70) >2 cm 9(32.14)* 61(66.30) 骨窗面积/cm2 139.70±43.38* 112.94±34.61 与非硬膜下积液组比较, *P < 0.05。 2.4 术后硬膜下积液影响因素的多因素Logistic回归分析
以“术后硬膜下积液”为因变量(赋值: 0=是, 1=否),以“中线移位、脑内血肿、蛛网膜撕裂、皮层切开、骨瓣边缘距中线距离、骨窗面积”为自变量纳入Logistic回归分析,赋值情况见表 4。多因素Logistic回归分析显示,中线移位、脑内血肿、蛛网膜撕裂、皮层切开、骨瓣边缘距中线距离、骨窗面积均为术后硬膜下积液的独立影响因素(P < 0.05), 见表 5。
表 4 术后硬膜下积液影响因素的多因素Logistic回归分析赋值情况变量 变量名 赋值方法 硬膜下积液 Y 是=0, 否=1 中线移位 X1 是=0, 否=1 脑内血肿 X2 是=0, 否=1 蛛网膜撕裂 X3 是=0, 否=1 皮层切开 X4 是=0, 否=1 骨瓣边缘距中线距离 X5 是=0, 否=1 骨窗面积 X6 变量参数 表 5 术后硬膜下积液影响因素的多因素Logistic回归分析变量 B S. E. Wald 自由度 P Exp(B) 95% CI 中线移位 -1.642 0.474 12.015 1 0.001 0.194 0.076~0.490 脑内血肿 -2.797 0.650 18.541 1 <0.001 0.061 0.017~0.218 蛛网膜撕裂 -1.678 0.647 6.724 1 0.010 0.187 0.052~0.664 皮层切开 -1.169 0.450 6.736 1 0.009 0.311 0.129~0.751 骨瓣边缘距中线距离 -1.424 0.461 9.549 1 0.002 0.241 0.098~0.594 骨窗面积 -0.019 0.006 9.601 1 0.002 0.981 0.969~0.993 3. 讨论
去骨瓣减压术是临床抢救脑伤患者的常用方案,能缓解脑内因水肿造成的高压,但易引发多种并发症,因此术后实行颅骨成形术非常重要[8-10]。本研究中,术后2个月研究组NIHSS评分低于对照组, Fugl-Meyer评分、Barthel评分高于术前与对照组,且研究组硬膜下积液、颅脑缺损综合征发生率低于对照组,提示早期行颅骨成形术有利于改善脑伤患者术后神经功能、运动功能与生活功能,且能有效减少并发症的发生,原因可能为[11-12]: ①脑损伤患者去骨瓣减压术后由于脑组织缺少骨瓣支撑,脑脊液循环、脑皮质灌注、血流动力学易发生异常,早期颅骨成形术能够避免长时间颅骨缺损,稳定颅内压,缓解血管压迫,改善血流循环。②去骨瓣减压术后使颅骨生理稳定性破坏,脑搏动使脑脊液进入硬膜下腔,早期颅骨修补能够使颅骨正常生理结构得以修复,消除颅内压力失衡状态。
既往研究[13]表明,去骨瓣减压术后高发硬膜下积液严重时可发展为脑积水、脑水瘤,严重威胁患者生命安全,因此分析其相关因素有利于预防术后硬膜下积液的发生。本研究显示,中线移位、脑内血肿、蛛网膜撕裂、皮层切开、骨瓣边缘距中线距离、骨窗面积均为术后硬膜下积液的独立影响因素,原因为[14-15]: ①蛛网膜与蝶骨脊紧密相连,蛛网膜撕裂发生于大脑裂缝或视交叉区域,缝蛛网膜撕裂易引发半球硬膜下积液,视交叉区域蛛网膜撕裂易致使纵裂积液; ②中线位移超过5 mm颅脑损伤较严重,手术性皮层切开易导致蛛网膜撕裂; ③脑内血肿影响脑脊液吸收途径; ④骨瓣边缘距中线距离小于2 cm的患者,脑桥静脉压力减小,脑静脉回流易发生障碍; ⑤骨窗面越大患者硬膜下、蛛网膜下及颅内存在梯度压力,使脑脊液吸收失衡,且压力越大,梯度压力越明显。
综上所述,脑外伤去骨瓣减压术联合早期颅骨成形术能有效改善患者神经功能、运动功能与日常生活功能,减少术后并发症的发生。中线移位、脑内血肿、蛛网膜撕裂、皮层切开、骨瓣边缘距中线距离、骨窗面积均为术后硬膜下积液的独立影响因素,临床可根据患者具体情况进行有效防治。
-
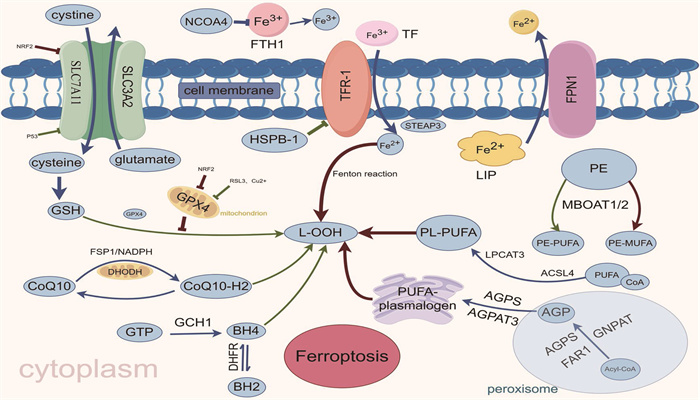
图 1 铁死亡主要调控机制
GSH: 谷胱甘肽; GSSG: 氧化型谷胱甘肽; GPX4: 谷胱甘肽过氧化物酶4; Glutamate: 谷氨酸;
SLC7A11: 溶质载体家族7成员11; SLC3A2: 溶质载体家族3成员2; Cysteine: 半胱氨酸; Cystine: 胱氨酸;
NRF2: 核因子E2相关因子2; L-OOH: 脂质过氧化物; L-OH: 脂质醇; CoQ10-H2: 还原形式的辅酶Q10;
FSP1: 铁死亡抑制蛋白1; DHODH: 二氢乳清酸脱氢酶; GCH1: 三磷酸鸟苷环化水解酶1; BH4: 四氢生物蝶呤;
BH2: 二氢生物蝶呤; GTP: 三磷酸鸟苷; DHFR: 二氢叶酸还原酶; MBOAT1/2: 膜结合O-酰基转移酶1/2;
MUFA: 单不饱和脂肪酸; PUFA: 多不饱和脂肪酸; ACSL4: 长链脂酰辅酶A合成酶4;
LPCAT3: 溶血卵磷脂酰基转移酶3; LIP: 不稳定铁池; TF: 转铁蛋白; TFR-1: 转铁蛋白受体1;
核NCOA4: 受体共激活因子4; FTH1: 铁蛋白重链1; HSPB1: 热休克蛋白β-; Acyl-CoA: 酰基辅酶A;
AGPS: 烷基甘油磷酸合成酶; FAR1: 脂肪酰基辅酶A还原酶1; GNPAT: 甘油磷酸O-酰基转移酶;
AGP: 前体1-O-烷基甘油-3-磷酸; AGPAT3: 1-酰基甘油-3-磷酸-O-酰基转移酶3;
PEDS1: 缩醛磷脂乙醇胺去饱和酶1; PUFA-plasmalogen: 多不饱和脂肪酸缩醛磷脂。 -
[1] PREM P N, SIVAKUMAR B, BOOVARAHAN S R, et al. Recent advances in potential of Fisetin in the management of myocardial ischemia-reperfusion injury-a systematic review[J]. Phytomedicine, 2022, 101: 154123. doi: 10.1016/j.phymed.2022.154123
[2] ZHAO W K, ZHOU Y, XU T T, et al. Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury[J]. Oxid Med Cell Longev, 2021, 2021: 9929687.
[3] YANG X, HUANG T Y, CHEN Y H, et al. Deoxynivalenol induces testicular ferroptosis by regulating the Nrf2/System Xc-/GPX4 axis[J]. Food Chem Toxicol, 2023, 175: 113730. doi: 10.1016/j.fct.2023.113730
[4] CUI Y, ZHANG Z L, ZHOU X, et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression[J]. J Neuroinflammation, 2021, 18(1): 249. doi: 10.1186/s12974-021-02231-x
[5] MA S X, SUN L Y, WU W H, et al. USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death[J]. Front Physiol, 2020, 11: 551318. doi: 10.3389/fphys.2020.551318
[6] XUE Q, YAN D, CHEN X, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis[J]. Autophagy, 2023, 19(7): 1982-1996. doi: 10.1080/15548627.2023.2165323
[7] LI W T, LIANG L, LIU S Y, et al. FSP1: a key regulator of ferroptosis[J]. Trends Mol Med, 2023, 29(9): 753-764. doi: 10.1016/j.molmed.2023.05.013
[8] MAO C, LIU X G, ZHANG Y L, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer[J]. Nature, 2021, 593(7860): 586-590. doi: 10.1038/s41586-021-03539-7
[9] HU Q, WEI W H, WU D Q, et al. Blockade of GCH1/BH4 axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis[J]. Front Cell Dev Biol, 2022, 10: 810327. doi: 10.3389/fcell.2022.810327
[10] CHEN Y F, LI X T, WANG S Y, et al. Targeting iron metabolism and ferroptosis as novel therapeutic approaches in cardiovascular diseases[J]. Nutrients, 2023, 15(3): 591. doi: 10.3390/nu15030591
[11] TIAN H, XIONG Y H, ZHANG Y, et al. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis[J]. Cell Stress Chaperones, 2021, 27(2): 149-164.
[12] WU H, LIU Q, SHAN X Y, et al. ATM orchestrates ferritinophagy and ferroptosis by phosphorylating NCOA4[J]. Autophagy, 2023, 19(7): 2062-2077. doi: 10.1080/15548627.2023.2170960
[13] FANG Y Y, CHEN X C, TAN Q Y, et al. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action[J]. ACS Cent Sci, 2021, 7(6): 980-989. doi: 10.1021/acscentsci.0c01592
[14] LIANG D G, FENG Y, ZANDKARIMI F, et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones[J]. Cell, 2023, 186(13): 2748-2764, e22. doi: 10.1016/j.cell.2023.05.003
[15] JIANG X J, STOCKWELL B R, CONRAD M. Ferroptosis: mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4): 266-282. doi: 10.1038/s41580-020-00324-8
[16] LIU J, KANG R, TANG D L. Signaling pathways and defense mechanisms of ferroptosis[J]. FEBS J, 2022, 289(22): 7038-7050. doi: 10.1111/febs.16059
[17] DOLL S, PRONETH B, TYURINA Y Y, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition[J]. Nat Chem Biol, 2017, 13(1): 91-98. doi: 10.1038/nchembio.2239
[18] TANG D L, KROEMER G. Peroxisome: the new player in ferroptosis[J]. Signal Transduct Target Ther, 2020, 5(1): 273. doi: 10.1038/s41392-020-00404-3
[19] 刘丹勇, 夏正远, 韩荣辉, 等. 心肌缺血再灌注损伤机制研究的回顾与展望[J]. 中国动脉硬化杂志, 2020, 28(12): 1013-1019. https://www.cnki.com.cn/Article/CJFDTOTAL-KDYZ202012002.htm [20] RODRIGO R, GONZÁLEZ-MONTERO J, SOTOMAYOR C G. Novel combined antioxidant strategy against hypertension, acute myocardial infarction and postoperative atrial fibrillation[J]. Biomedicines, 2021, 9(6): 620. doi: 10.3390/biomedicines9060620
[21] ZINDEL J, KUBES P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation[J]. Annu Rev Pathol, 2020, 15: 493-518. doi: 10.1146/annurev-pathmechdis-012419-032847
[22] WANG J, LIU Y, LIU Y, et al. Recent advances in nanomedicines for imaging and therapy of myocardial ischemia-reperfusion injury[J]. J Control Release, 2023, 353: 563-590. doi: 10.1016/j.jconrel.2022.11.057
[23] HEUSCH G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective[J]. Nat Rev Cardiol, 2020, 17(12): 773-789. doi: 10.1038/s41569-020-0403-y
[24] IBÁÑEZ B, HEUSCH G, OVIZE M, et al. Evolving therapies for myocardial ischemia/reperfusion injury[J]. J Am Coll Cardiol, 2015, 65(14): 1454-1471. doi: 10.1016/j.jacc.2015.02.032
[25] SPARVERO L J, TIAN H, AMOSCATO A A, et al. Direct mapping of phospholipid ferroptotic death signals in cells and tissues by gas cluster ion beam secondary ion mass spectrometry (GCIB-SIMS)[J]. Angew Chem Int Ed, 2021, 60(21): 11784-11788. doi: 10.1002/anie.202102001
[26] TANG L J, LUO X J, TU H, et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion[J]. Naunyn Schmiedebergs Arch Pharmacol, 2021, 394(2): 401-410. doi: 10.1007/s00210-020-01932-z
[27] YAN H F, ZOU T, TUO Q Z, et al. Ferroptosis: mechanisms and links with diseases[J]. Signal Transduct Target Ther, 2021, 6(1): 49. doi: 10.1038/s41392-020-00428-9
[28] LV Z Q, WANG F E, ZHANG X F, et al. Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via Nrf2/HO-1 pathway[J]. Shock, 2021, 56(3): 440-449. doi: 10.1097/SHK.0000000000001751
[29] KWON M Y, PARK E, LEE S J, et al. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death[J]. Oncotarget, 2015, 6(27): 24393-24403. doi: 10.18632/oncotarget.5162
[30] FAN Z Y, CAI L L, WANG S N, et al. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis[J]. Front Pharmacol, 2021, 12: 628988. doi: 10.3389/fphar.2021.628988
[31] LI W Y, LI W, WANG Y, et al. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury[J]. Cell Death Discov, 2021, 7(1): 267. doi: 10.1038/s41420-021-00656-0
[32] LEI D Y, LI B, ISA Z, et al. Hypoxia-elicited cardiac microvascular endothelial cell-derived exosomal miR-210-3p alleviate hypoxia/reoxygenation-induced myocardial cell injury through inhibiting transferrin receptor 1-mediated ferroptosis[J]. Tissue Cell, 2022, 79: 101956. doi: 10.1016/j.tice.2022.101956
[33] TANG L J, ZHOU Y J, XIONG X M, et al. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion[J]. Free Radic Biol Med, 2021, 162: 339-352. doi: 10.1016/j.freeradbiomed.2020.10.307
[34] LAKHAL-LITTLETON S, WOLNA M, CHUNG Y J, et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis[J]. Elife, 2016, 5: e19804. doi: 10.7554/eLife.19804
[35] QIU M L, YAN W, LIU M M. YAP facilitates NEDD4L-mediated ubiquitination and degradation of ACSL4 to alleviate ferroptosis in myocardial ischemia-reperfusion injury[J]. Can J Cardiol, 2023, 39(11): 1712-1727. doi: 10.1016/j.cjca.2023.07.030
[36] CAI W B, LIU L, SHI X L, et al. Alox15/15-HpETE aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis[J]. Circulation, 2023, 147(19): 1444-1460. doi: 10.1161/CIRCULATIONAHA.122.060257
[37] LEE Y S, LEE D H, CHOUDRY H A, et al. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis[J]. Mol Cancer Res, 2018, 16(7): 1073-1076. doi: 10.1158/1541-7786.MCR-18-0055
[38] LI W Y, LI W, LENG Y, et al. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress[J]. DNA Cell Biol, 2020, 39(2): 210-225. doi: 10.1089/dna.2019.5097
[39] ZHOU Y Q, ZHOU H X, HUA L, et al. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis[J]. Free Radic Biol Med, 2021, 171: 55-68. doi: 10.1016/j.freeradbiomed.2021.05.009
[40] ZHAO K, CHEN X S, BIAN Y J, et al. Broadening horizons: The role of ferroptosis in myocardial ischemia-reperfusion injury[J]. Naunyn Schmiedebergs Arch Pharmacol, 2023, 396(10): 2269-2286. doi: 10.1007/s00210-023-02506-5
[41] XU J F, ZHANG M H, LIU F, et al. Mesenchymal stem cells alleviate post-resuscitation cardiac and cerebral injuries by inhibiting cell pyroptosis and ferroptosis in a swine model of cardiac arrest[J]. Front Pharmacol, 2021, 12: 793829. doi: 10.3389/fphar.2021.793829
[42] YAN N, XU Z P, QU C H, et al. Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway[J]. Int Immunopharmacol, 2021, 98: 107844. doi: 10.1016/j.intimp.2021.107844
-
期刊类型引用(1)
1. 郭英惠,代红燕,姚雪萍,任芳,尹娜,左晓婷. Klotho蛋白对大鼠心肌缺血-再灌注损伤的作用机制探究. 现代生物医学进展. 2024(19): 3616-3618+3644 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号
